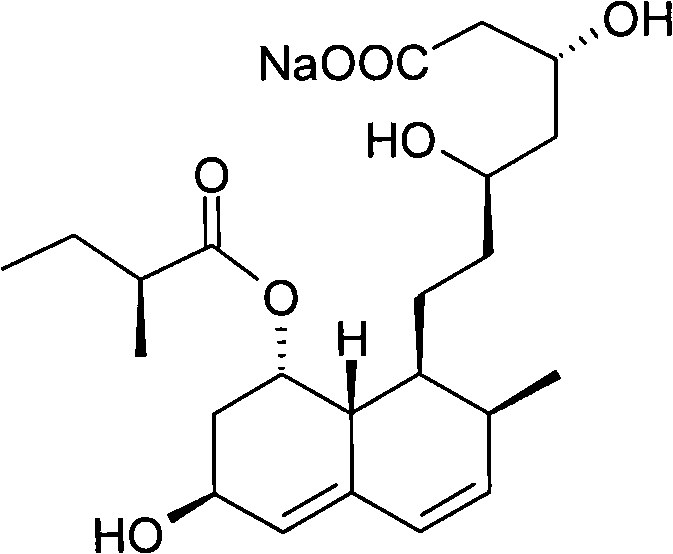
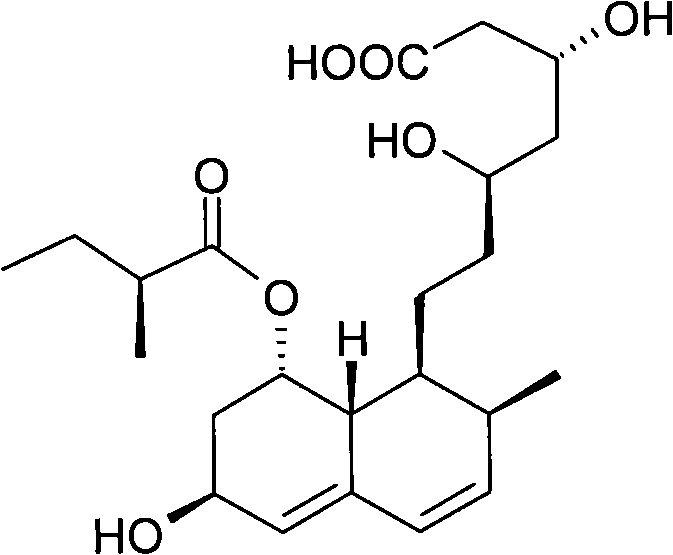
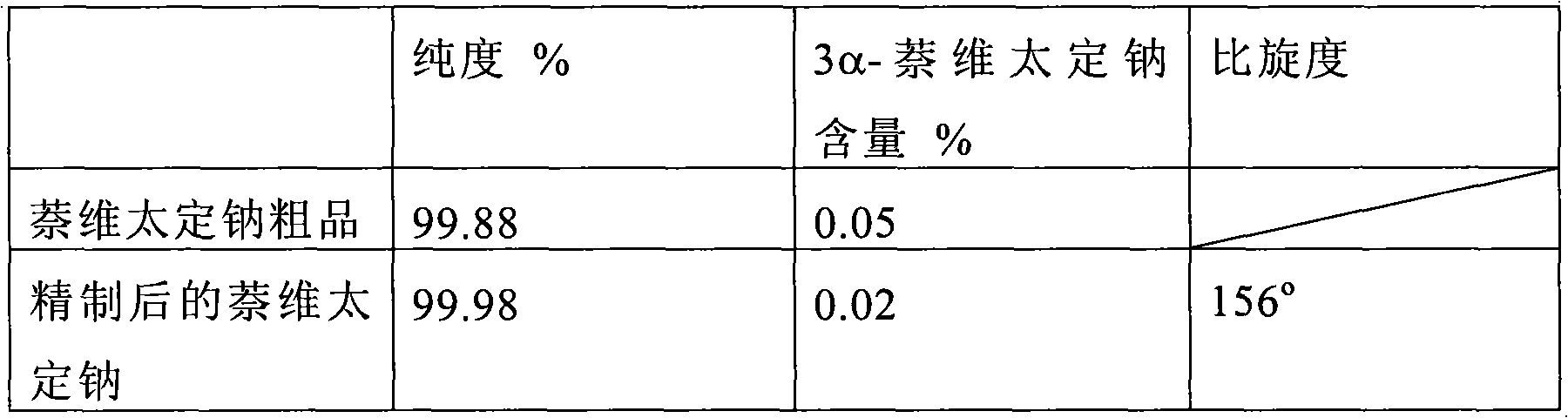
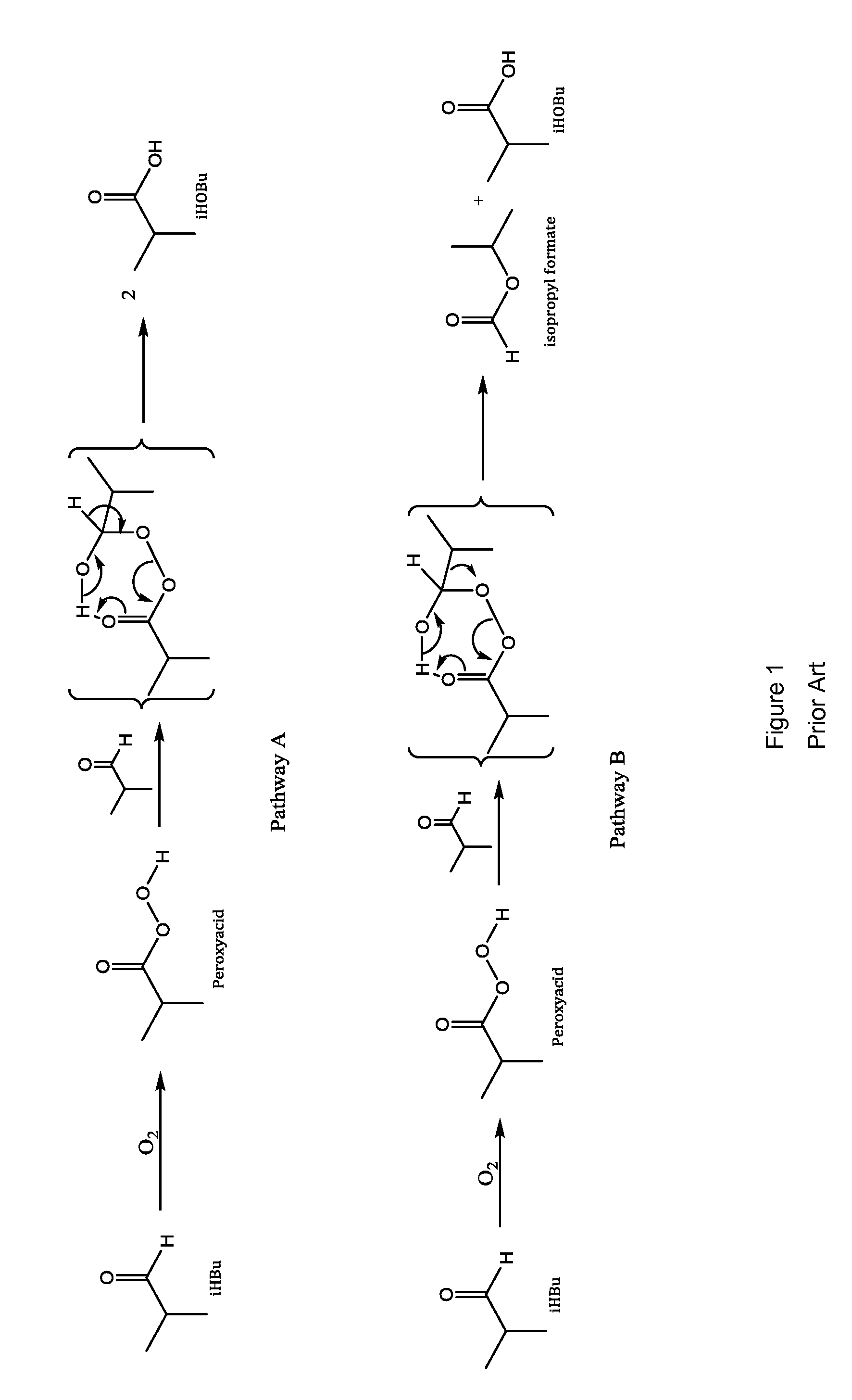
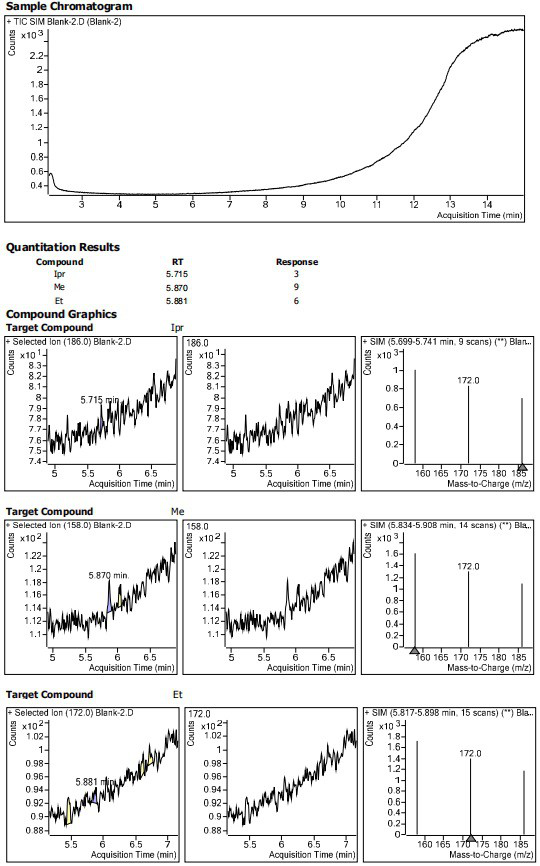
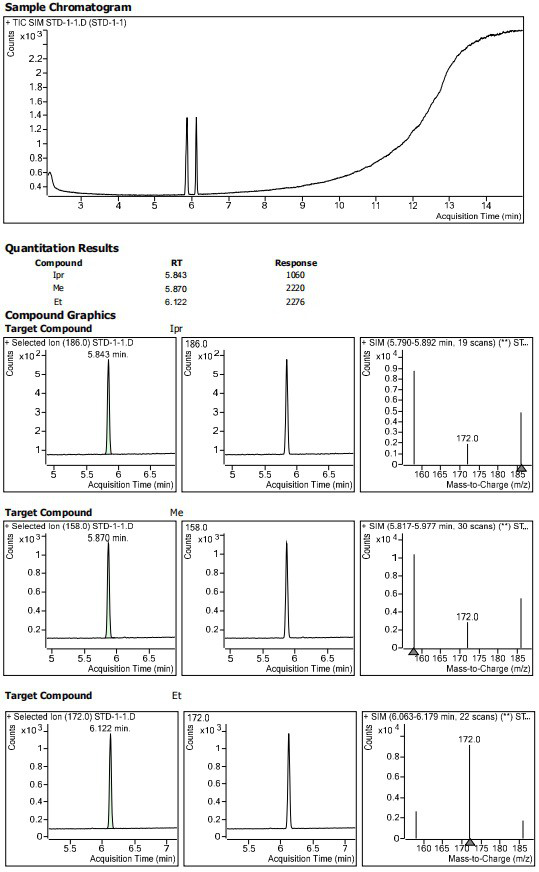
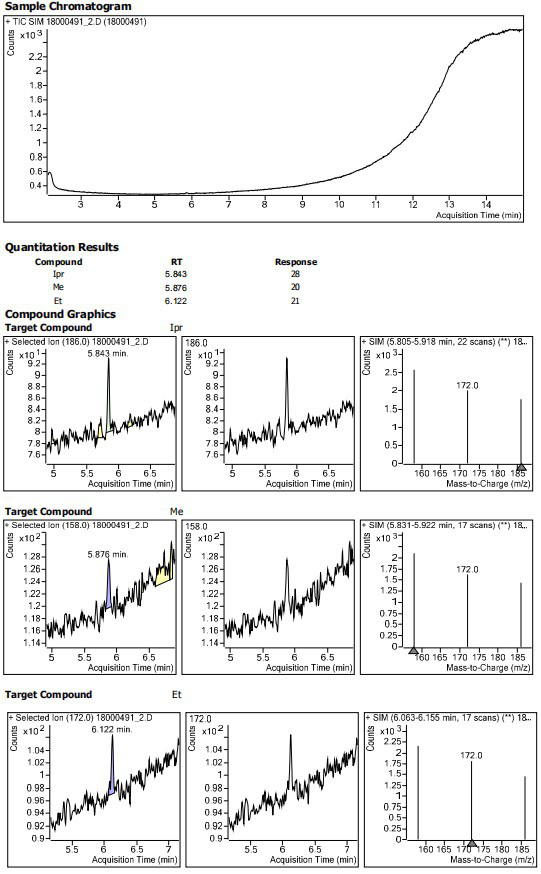
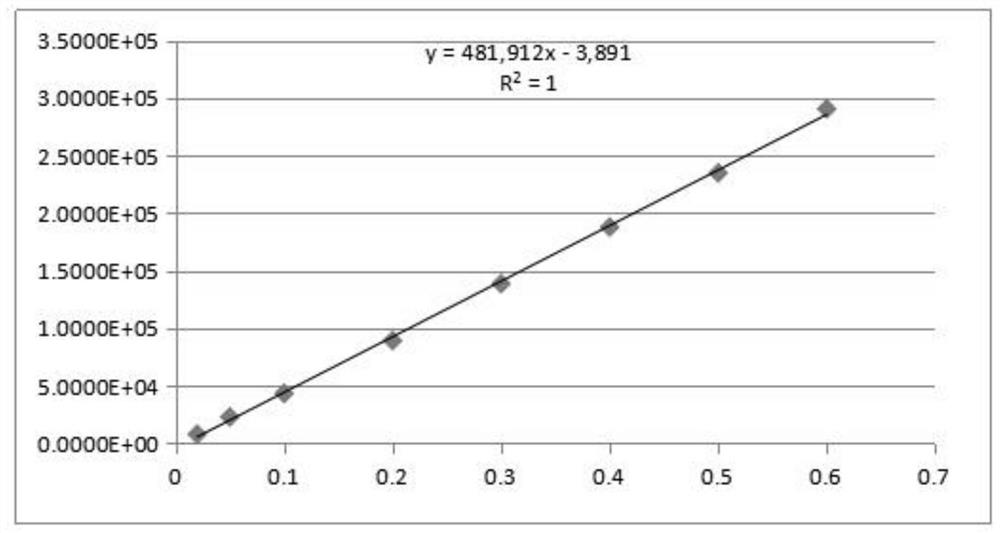
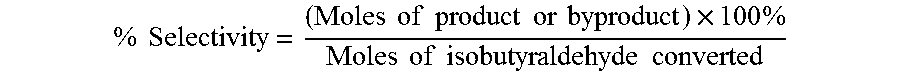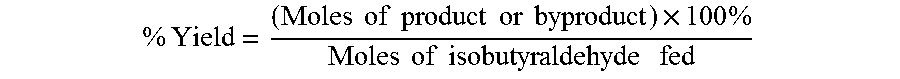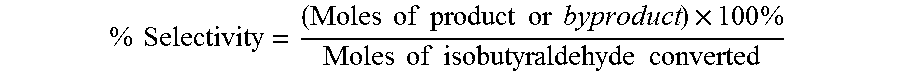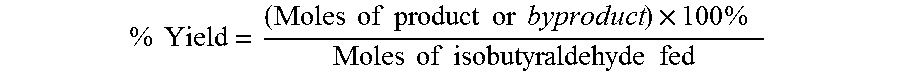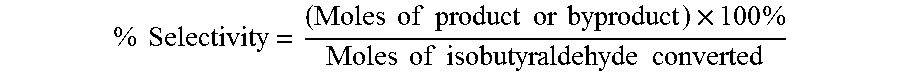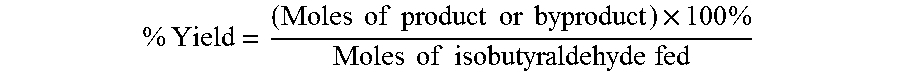Patents
Literature
33 results about "Isopropyl formate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isopropyl formate is expected to be mobile in soil. Although data specific to isopropyl formate are unavailable, its structure suggests that it will biodegrade readily in soil or water. Occupational exposure occurs through inhalation of vapors and through dermal contact.
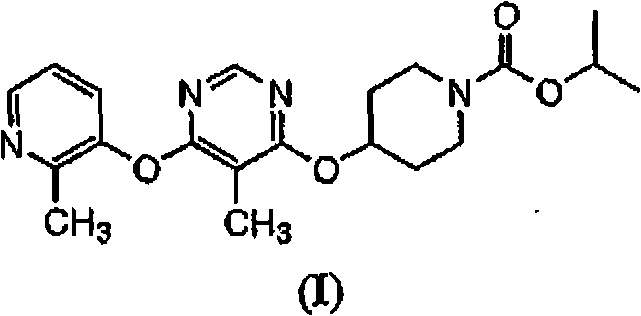
Modulators of metabolism and the treatment of disorders related thereto
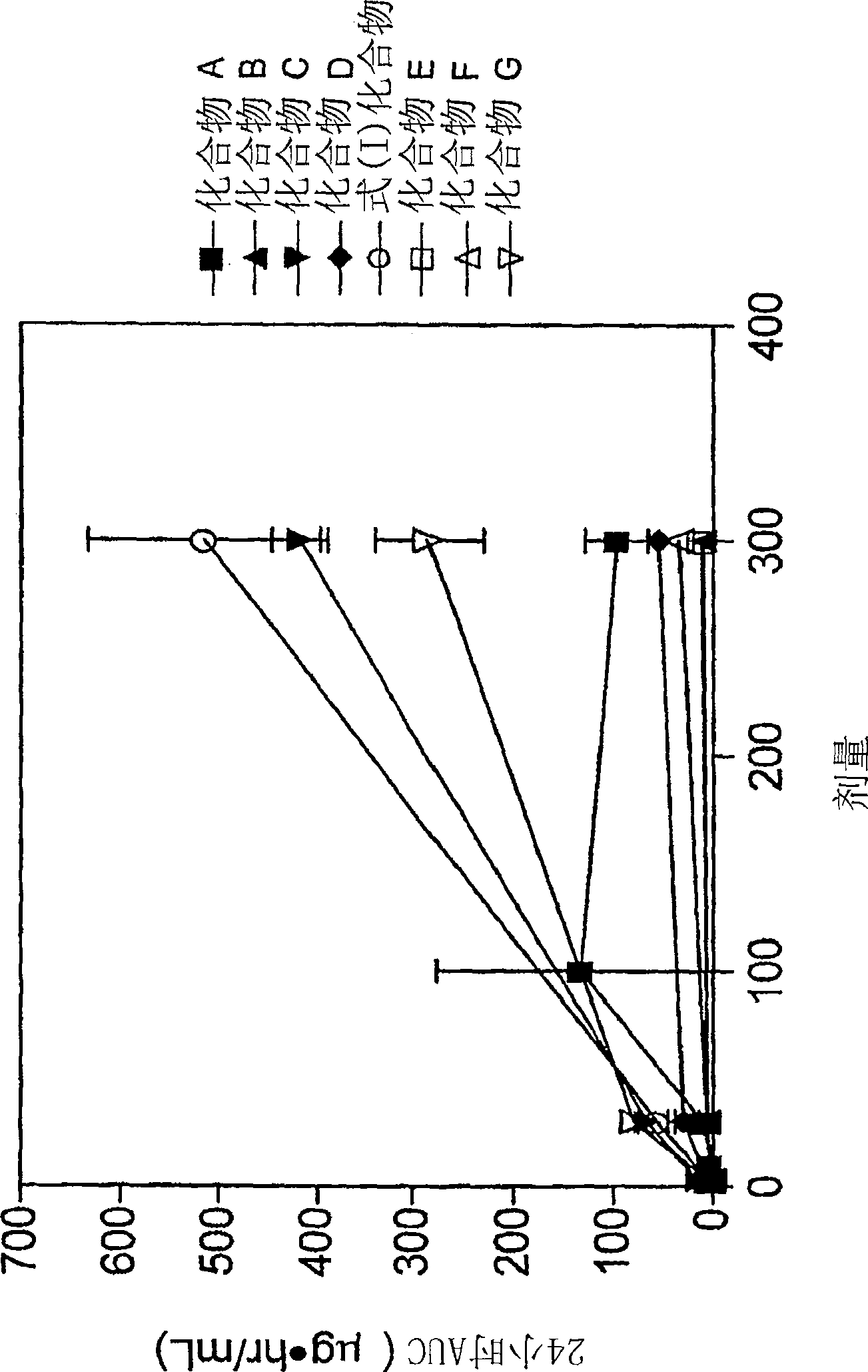
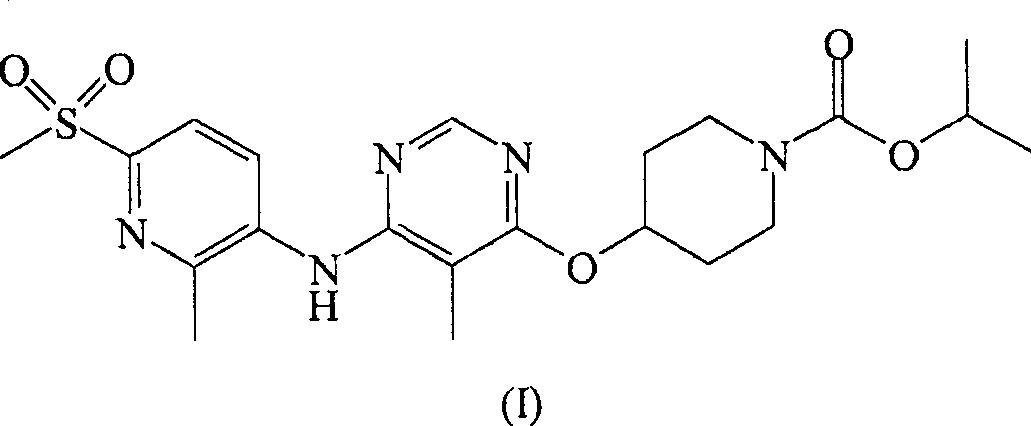
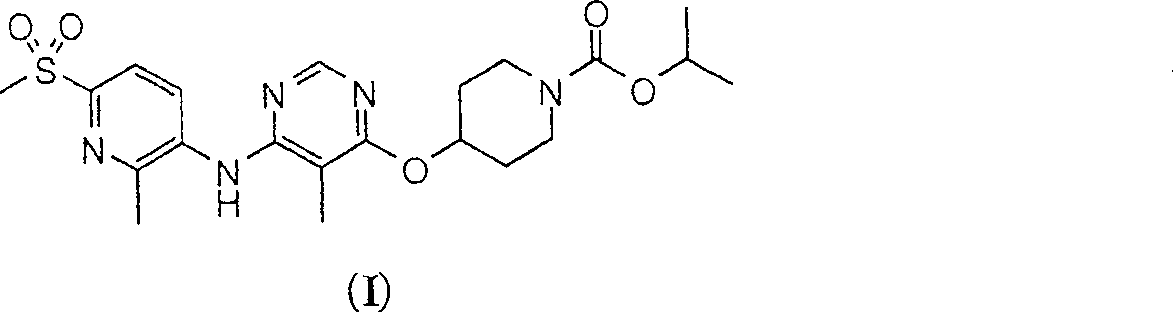
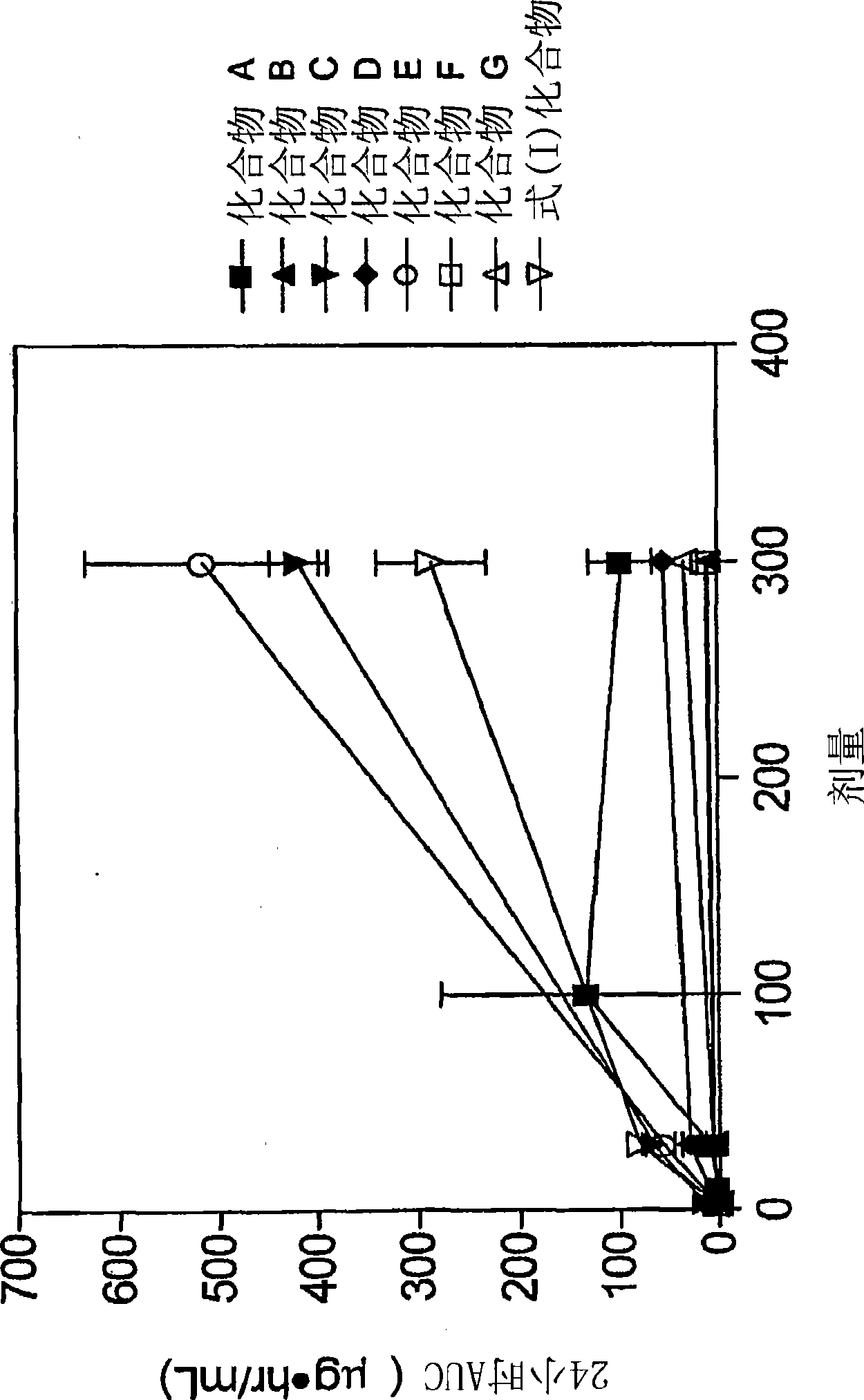
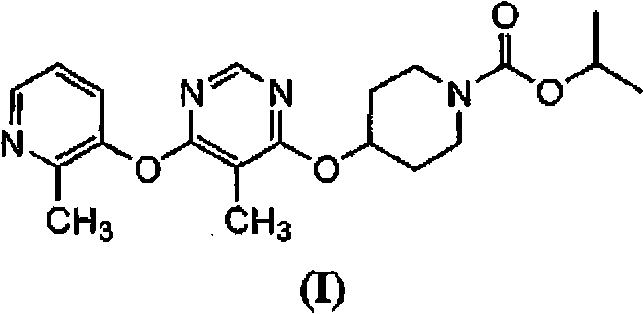
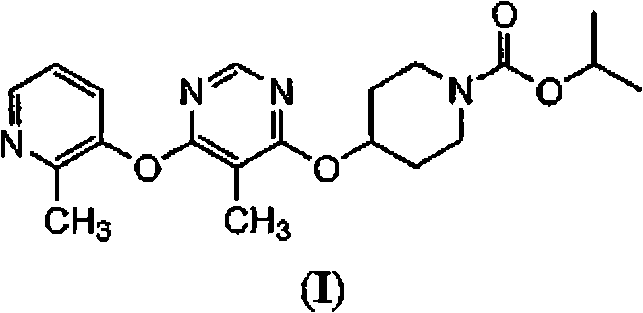
The present invention relates to 4-[6-(6-methanesulfonyl-2-methyl-pyridin-3-ylamino)-5-methyl-pyrimidin-4-yloxy]-piperidine-1-carboxylic acid isopropyl ester, pharmaceutically acceptable salts, solvates and hydrates thereof that are modulators of glucose metabolism. Accordingly, compounds of the present invention are useful in the treatment of metabolic-related disorders and complications thereof, such as, diabetes and obesity.
Owner:ARENA PHARMA
Modulators of metabolism and the treatment of disorders related thereto
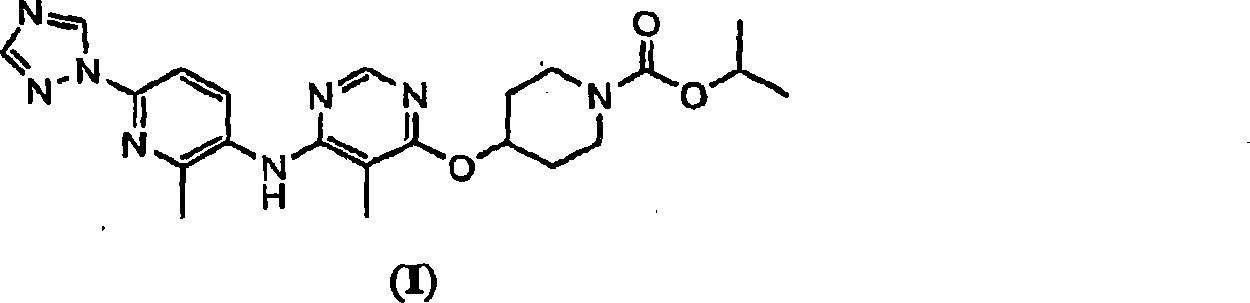
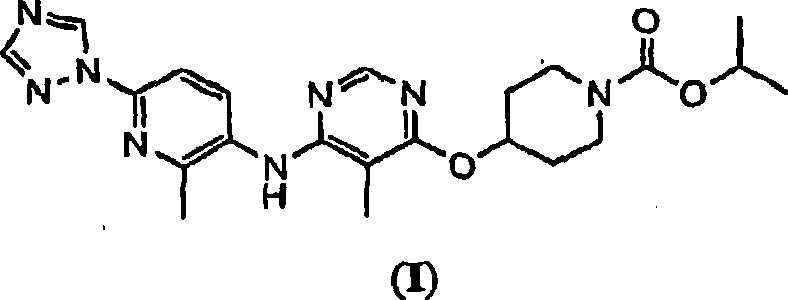
The present invention relates to 4-[5-Methoxy-6-(2-methyl-6-[1,2,4]triazol-1-yl-pyridin-3-ylamino)-pyrimidin-4-yloxy]-piperidine-1-carboxylic acid isopropyl ester, pharmaceutically acceptable salts, solvates and hydrates thereof that are modulators of glucose metabolism. Accordingly, compounds of the present invention are useful in the treatment of metabolic-related disorders and complications thereof, such as, diabetes and obesity.
Owner:ARENA PHARMA
Pesticidal composition and method for controlling pests
InactiveCN1620250AStable and efficient pest control effectBiocideDead animal preservationFungicideThiazole
Pesticide composition comprising as active ingredients at least one specific imidazole compound and at least one fungicide, wherein the fungicide is selected from (S)-5-methyl-2-methylthio-5- Phenyl-3-phenylamino-3,5-dihydroimidazol-4-one, 2-methyl-1-[(1-p-tolylethyl)carbamoyl]-(S)-propylamino Isopropyl formate, 3,5-dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-4-methylbenzamide and N-(α- Cyano-2-thienyl)-4-ethyl-2-(ethylamino)-5-thiazolecarbamoyl.
Owner:ISHIHARA SANGYO KAISHA LTD
Pharmaceutical composition for treating obesity
The invention discloses a pharmaceutical composition for treating obesity-related diseases. The composition contains a therapeutically-effective amount of 1-isopropyl-1'-(1-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-5-carbonyl)-4,6-dihydrospiro[indazol-5,4'-piperidine]-7(1H)-one and a therapeutically-effective amount of 4-[5-cyano-4-({2-fluoro-4-[(2-hydroxyethyl) sulfonyl]phenoxy}methyl)-1H-pyrazol-1-yl]piperidin-1-isopropyl formate which have the mass ratio of (3: 1) to (1: 3). By using the two kinds of drugs in a combined manner, symptoms, such as hypertension and diabetes, caused by obesity can be treated while the obesity is treated.
Owner:WUXI XINDA MEDICAL DEVICE
Preparation method of crystal form I linezolid
InactiveCN102850290AReduce pollutionMixed crystal is effectiveOrganic chemistryAcetic acidOrganic solvent
The invention relates to a preparation method of crystal form I linezolid. The method comprises the steps of: adding a linezolid crude product, non-crystal form I linezolid or mixed linezolid crystal into an ester or toluene solvent, wherein the ester solvent is any one selected from ethyl acetate, acetic acid isobutyl ester, tert-butyl acetate, acetic acid isopropyl ester, isopropyl formate, or ethyl formate, and the amount ratio of the ester or toluene solvent to the linezolid crude product, the non-crystal form I linezolid or the mixed linezolid crystal is (5-40mL): 1g; heating and dissolving the linezolid crude product, the non-crystal form I linezolid or the mixed linezolid crystal into the solvent, and cooling to separate out crystal; filtering the mixed liquid; and finally, washing a filter cake by an organic solvent and drying to obtain the crystal form I linezolid. The preparation method of the crystal form I linezolid is less in solvent amount, does not need crystal nucleus induction, only needs dissolving by heating, cooling and filtering, and is simple and convenient to operate and mild in conditions, thus the preparation method is suitable for industrial production; meanwhile, the method is high in yield.
Owner:TIANJIN WEIJIE TECH
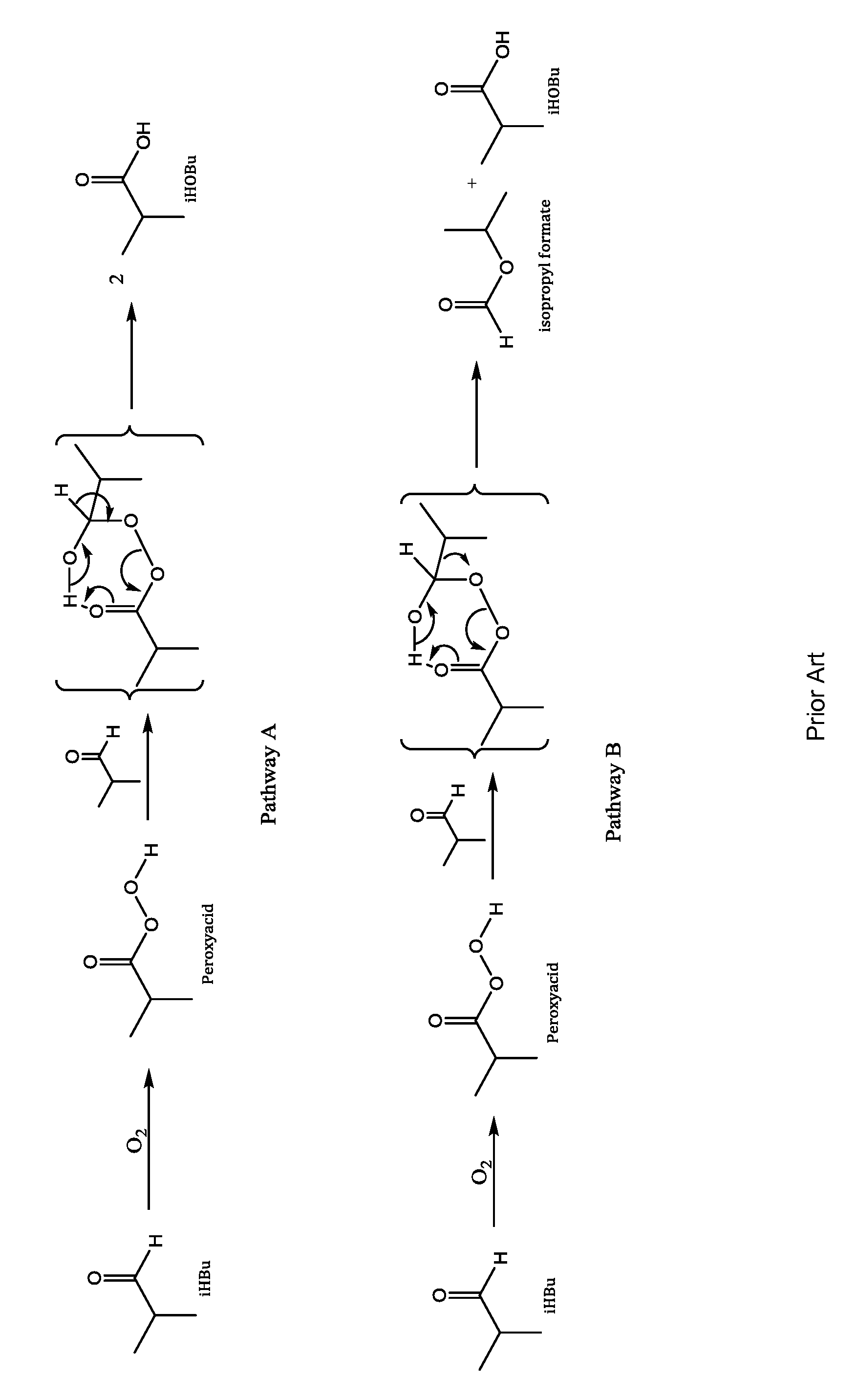
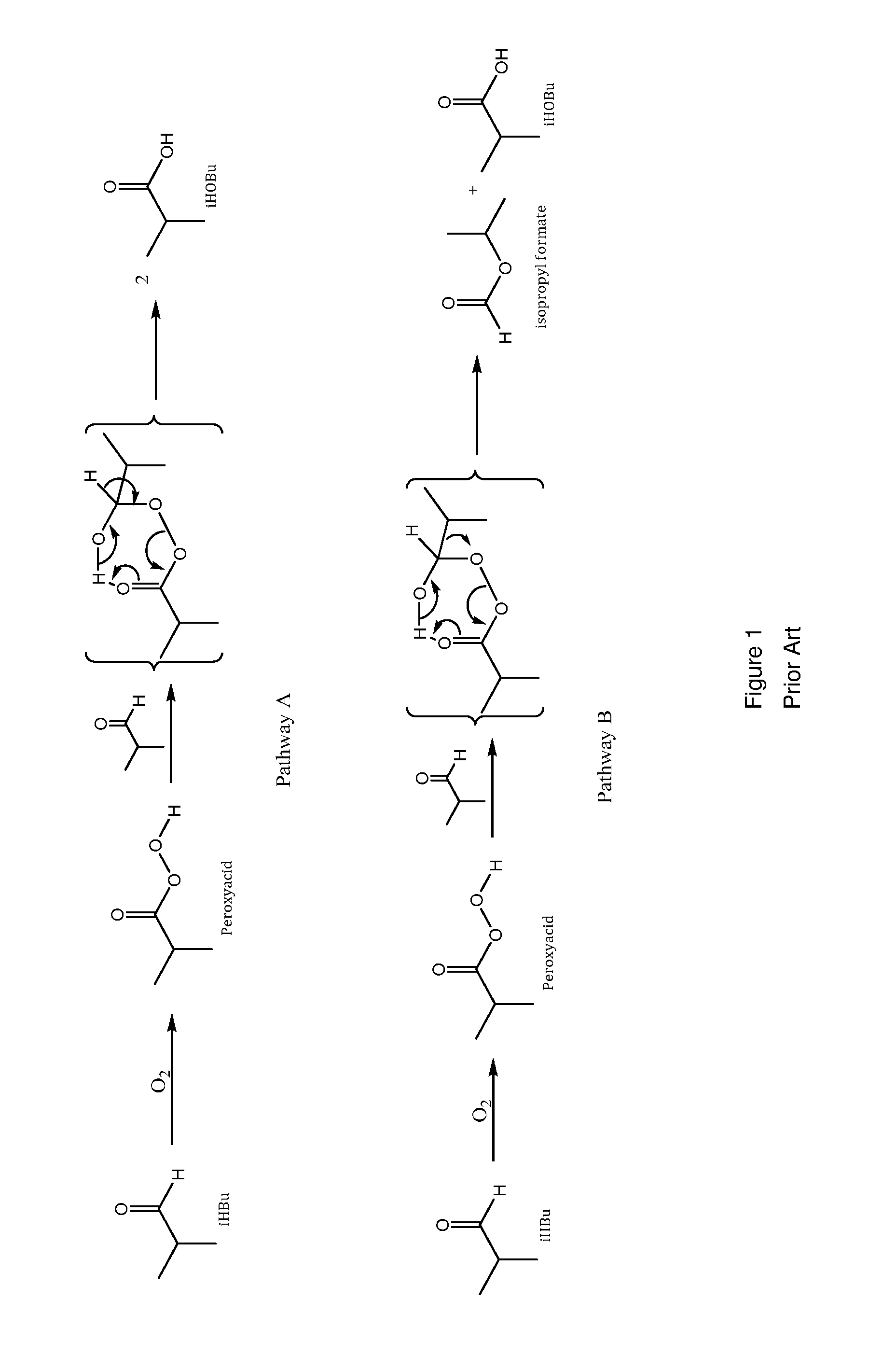
Aldehyde oxidation processes
ActiveUS9428435B2Reduce formationOrganic compound preparationPreparation by aldehyde oxidation-reductionStatic mixerIsopropyl formate
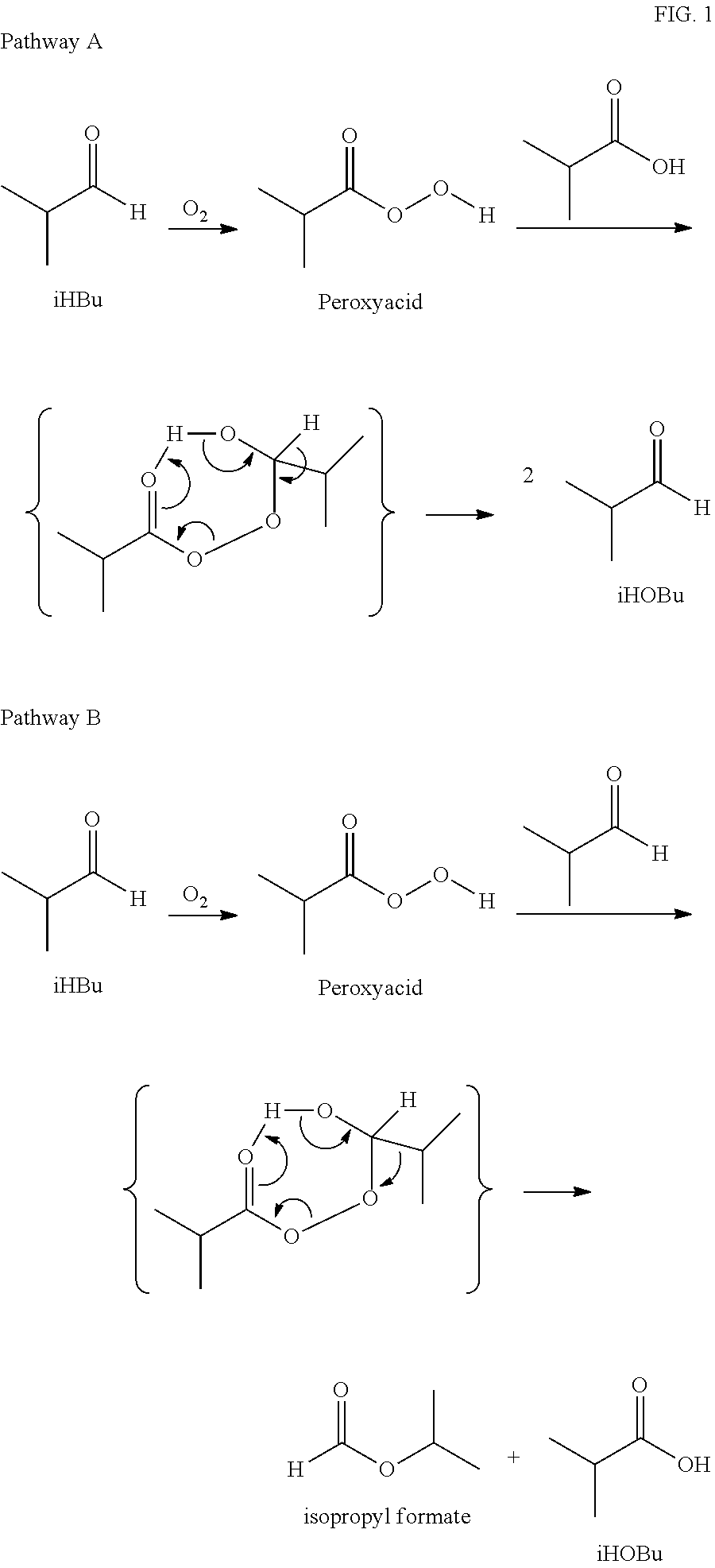
The oxidation of isobutyraldehyde produces isobutyric acid and byproducts, such as isopropyl formate. A process of reducing the isopropyl formate byproduct and other byproducts in the oxidation of isobutyraldehyde is described. The process uses a carbonyl compound, such as acetone, to reduce byproduct levels in the resulting product. Process for use of static mixers in oxidation reactions of aldehydes are also provided.
Owner:EASTMAN CHEM CO
SBS grafted odor-removing mattress spraying glue and preparation method thereof
ActiveCN107177332AIncrease forceReduce the numberMacromolecular adhesive additivesGraft polymer adhesivesElastomerMethyl isobutyl ketone
Owner:佛山市东方树新材料科技有限公司
Reduction of ester formation in isobutyraldehyde oxidation
ActiveUS20150166450A1Reduce formationOrganic compound preparationCarboxylic preparation by oxidationProduction rateIsopropyl formate
The oxidation of isobutyraldehyde produces isobutyric acid and byproducts, such as isopropyl formate. A method of reducing the isopropyl formate byproduct in the oxidation of isobutyraldehyde is described. The method uses a co-solvent, such as acetone, to the isobutyraldehyde feed to increase both the selectivity of the reaction to isobutyric acid and the production rate of isobutyric acid so that the isopropyl formate byproduct is significantly reduced.
Owner:EASTMAN CHEM CO
Preparation method of azelnidipine
InactiveCN106279109AGreat purification difficultyLow yieldOrganic chemistryAcetic acidDihydropyridine
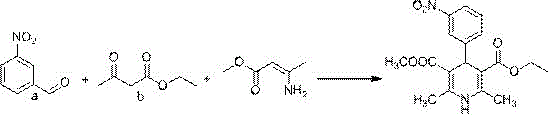
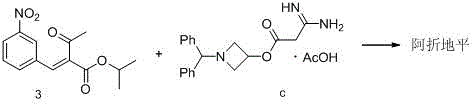
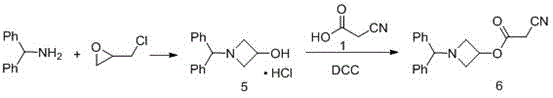
The invention relates to a preparation method of azelnidipine, belongs to the field of medicines and opens up a new way for synthesizing dihydropyridine calcium antagonists. With cyanoacetic acid as a raw material, the method comprises the following sequential steps: synthesizing isopropyl 2-amino-3-carboxyl-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-5-formate through a Pinner reaction and a Hantzsch reaction; and performing DCC dehydration esterification with 1-diphenylmethyl-3-hydroxyl azetidine hydrochloride to obtain a crude product of azelnidipine; and performing further crystal transformation to obtain alpha-crystal type azelnidipine.
Owner:WEIHAI DISU PHARMA CO LTD +1
Pesticidal composition and method for controlling pests
InactiveCN1284454CStable and efficient pest control effectBiocideDead animal preservationEthyl groupAcyl group
Pesticide composition comprising as active ingredients at least one specific imidazole compound and at least one fungicide, wherein the fungicide is selected from (S)-5-methyl-2-methylthio-5- Phenyl-3-phenylamino-3,5-dihydrimidazol-4-one, 2-methyl-1-[(1-p-tolylethyl)carbamoyl]-(S)-propylamino Isopropyl formate, 3,5-dichloro-N-(3-chloro-1-ethyl-1-methyl-2-oxopropyl)-4-methylbenzamide and N-(α- Cyano-2-thienyl)-4-ethyl-2-(ethylamino)-5-thiazolecarbamoyl.
Owner:ISHIHARA SANGYO KAISHA LTD
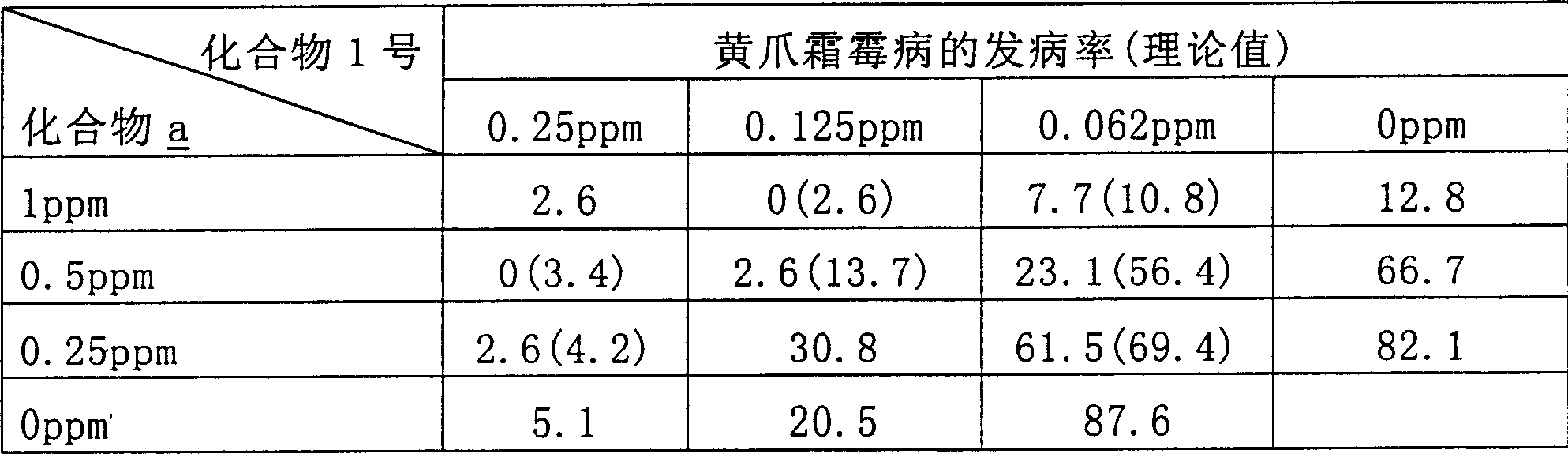
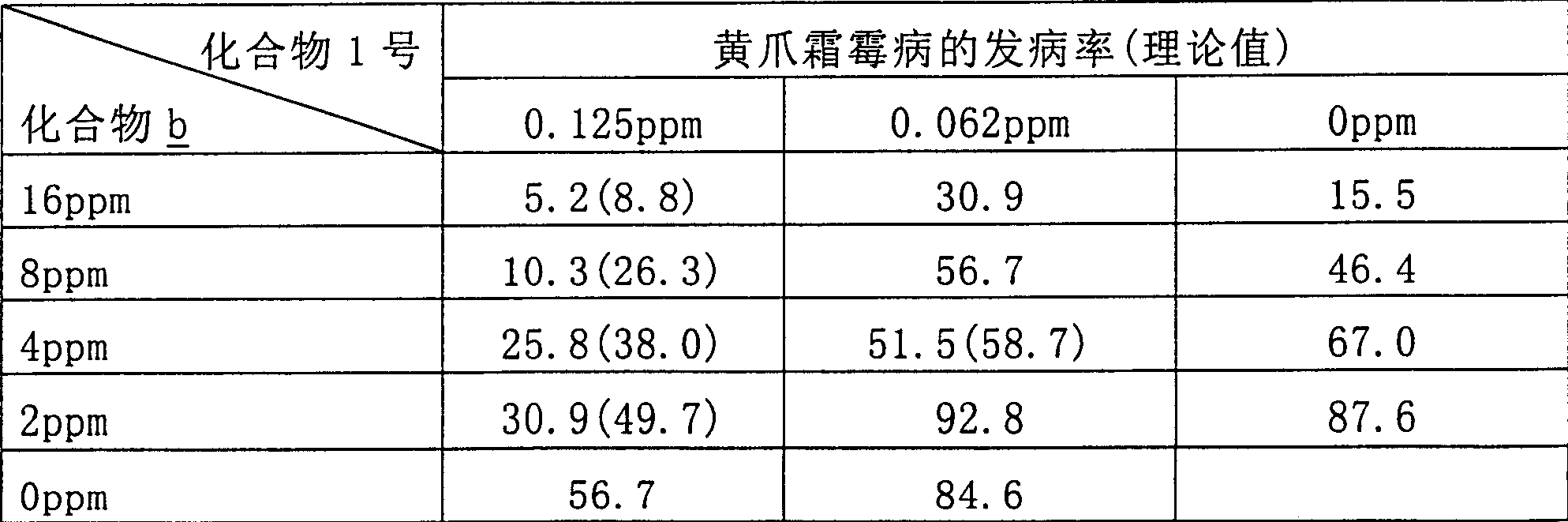
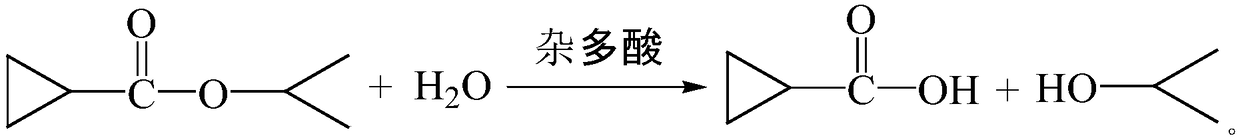
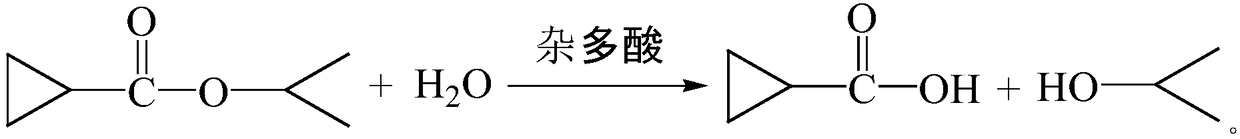
Method for preparing cyclopropanecarboxylic acid by using heteropolyacid catalyst
InactiveCN109280003AGood choiceAvoid it happening againPreparation from carboxylic acid esters/lactonesDistillationRoom temperature
The invention provides a method for preparing cyclopropanecarboxylic acid by using a heteropolyacid catalyst. The method comprises the following steps: adding 192g of isopropyl cyclopropanecarboxylateinto a flask, adding 54g of water, uniformly carrying out stirring, adding 1-10% of the heteropolyacid catalyst, uniformly carrying out stirring, then carrying out a reaction at room temperature for2 hours, carrying out filtering after the reaction is ended, collecting the heteropolyacid catalyst for reuse, carrying out normal-pressure distillation on the obtained mother liquor, and collecting fractions at a temperature of 80-82 DEG C to obtain the cyclopropanecarboxylic acid. According to the invention, the catalyst with relatively good selectivity is selected, so that generation of a largeamount of salt-containing wastewater can be avoided, the catalyst can be reused and can be easily separated from the system, the yield is relatively high, and the novel catalyst adopted in the process has low corrosion to equipment.
Owner:DAFENG YUELONG CHEM
Structural filter core for removing benzene from drinking water and preparation method thereof
InactiveCN102463002AHigh removal rateNo secondary pollutionWater contaminantsFiltration separationBenzenePotable water
The invention discloses structural filter cores and specifically relates to a structural filter core for removing benzene from drinking water and a preparation method thereof. In the preparation process of the structural filter core, a certain amount of 180-mesh bentonite is weighed according to ratio of raw materials, sodium sulfide is added in the bentonite, and activation is carried out for 120-240min at the temperature of 120-180 DEG C; the activated mixture is mixed with polyvinyl chloride, a certain amount of azodicarboxylate isopropyl is then added and mixed to be even; and the mixture is put in a columnar mold, sintered at the temperature of 320-400 DEG C and molded after about 210-300min, and demolded till being cooled by airing, and then, the columnar filter core with the pore diameter of 3mm to 5mm and the porosity factor of 30% to 40% is formed. The structural filter core not only purifies the drinking water, but also avoids the injury to human bodies arising from excessive benzene in the drinking water, thereby being very applicable to the treatment of drinking water at home terminals. Proved by test, the structural filter core can have a ratio of over 99.9% in the removal of the benzene in the drinking water.
Owner:CHANGZHOU YAHUAN ENVIRONMENTAL PROTECTION TECH
Application of composition in preparation of medicine for treating obesity
The invention discloses application of a composition in preparation of a medicine for treating obesity. The composition contains a therapeutically-effective amount of 1-isopropyl-1'-(1-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-5-carbonyl)-4,6-dihydrospiro[indazol-5,4'-piperidine]-7(1H)-one and a therapeutically-effective amount of 4-[5-cyano-4-({2-fluoro-4-[(2-hydroxyethyl)sulfonyl]phenoxy}methyl)-1H-pyrazol-1-yl]piperidin-1-isopropyl formate which have the mass ratio of (3: 1) to (1: 3). By using the two kinds of drugs in a combined manner, symptoms, such as hypertension and diabetes, caused by the obesity can be treated while the obesity is treated.
Owner:WUXI XINDA MEDICAL DEVICE
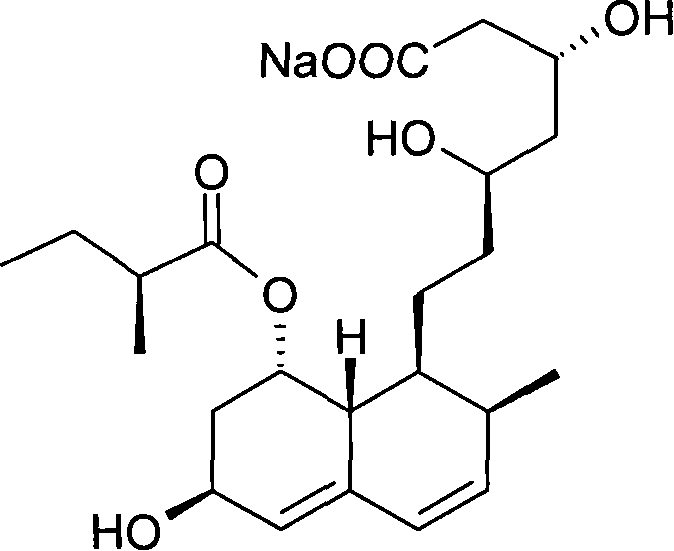
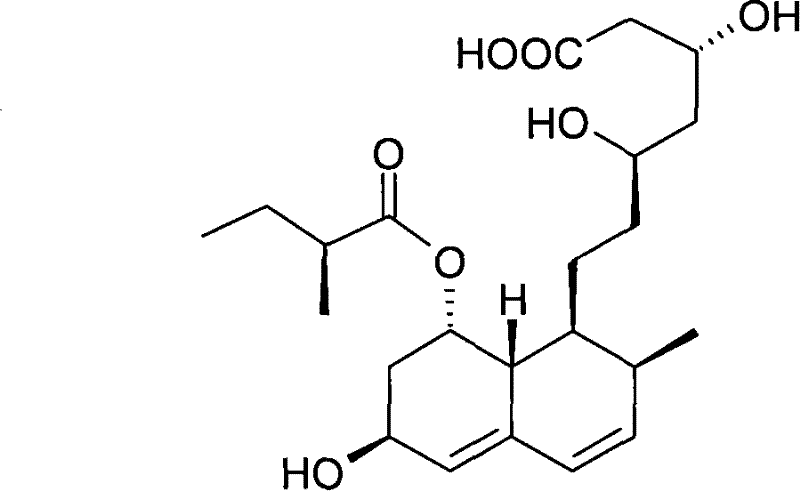
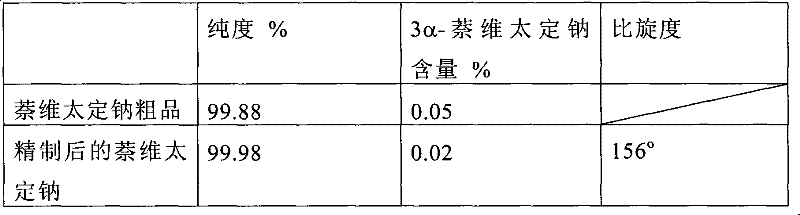
Preparation method of sodium salt of pravastatin
ActiveCN101580466BReduce usageLow facility requirementsOrganic compound preparationMetabolism disorderIsopropyl formateSodium salt
The invention discloses a preparation method of sodium salt of pravastatin, which comprises the following steps: adding inorganic alkaline or organic alkaline containing sodium ions as positive ions into organic solution containing the pravastatin until pH is more than 7 and obtaining sodium pravastatin; the organic solution containing the pravastatin comprises the pravastatin and first solvent, wherein the first solvent is selected from one or a plurality of methyl formate, n-propyl formate, isopropyl formate, n-butyl formate, methyl acetate, ethyl acetate, n-propyl acetate, isopropyl acetate, n-butyl acetate, sec-butyl acetate, isobutyl acetate and tert-butyl acetate.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Modulators of metabolism and the treatment of disorders related thereto
InactiveCN101263135AReserve exclusionOrganic active ingredientsOrganic chemistryDiabetes mellitusGlucose polymers
The present invention relates to 4-[5-methyl-6-(2-methyl-pyridin-3-yloxy)-pyrimidin-4-yloxy]-piperidine-1-carboxylic acid isopropyl ester, pharmaceutically acceptable salts, solvates and hydrates thereof that are modulators of glucose metabolism. Accordingly, compounds of the present invention are useful in the treatment of metabolic-related disorders and complications thereof, such as, diabetes and obesity.
Owner:ARENA PHARMA
Pharmaceutical composition for treating type 2 diabetes
InactiveCN104274446AEffective treatmentOrganic active ingredientsMetabolism disorderMedicineIsopropyl formate
The invention discloses a pharmaceutical composition for treating type 2 diabetes. The pharmaceutical composition contains a therapeutically-effective amount of 1'-(1H-indazol-5-carbonyl)-1-isopropyl-4,6-dihydrospiro[indazol-5,4'-piperidine]-7(1H)-one and a therapeutically-effective amount of 4-[5-cyano-4-({2-fluoro-4-[1-(2-hydroxyethyl)-1H-tetrazol-5-yl]phenoxy}methyl)-1H-pyrazol-1-yl]piperidin-1-isopropyl formate which have the mass ratio of (3: 1) to (1: 3). The pharmaceutical composition can be used for effectively treating the type 2 diabetes.
Owner:WUXI XINDA MEDICAL DEVICE
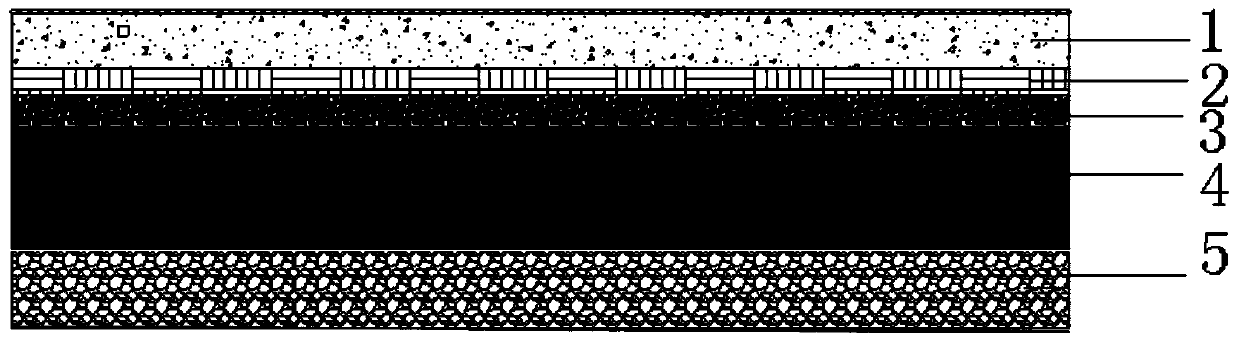
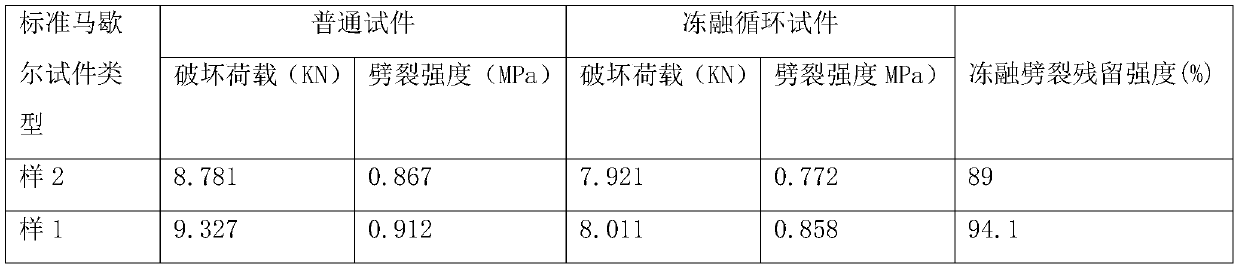
Asphalt concrete snow-melting and ice-inhibiting pavement structure
PendingCN110939035AImprove durabilityImprove road performanceIn situ pavingsPaving detailsEpoxyBenzoic acid
The invention discloses an asphalt concrete snow-melting and ice-inhibiting pavement structure. The pavement structure sequentially comprises a snow-melting and ice-inhibiting asphalt concrete surfacelayer, an impermeable layer, a stress absorption layer, a gravel layer and a base layer from top to bottom, wherein the snow-melting and ice-inhibiting asphalt concrete surface layer is formed by mixing an asphalt mixture and a snow-melting and ice-inhibiting material and paving; the stress absorption layer is formed by mixing and paving rubber and asphalt; wherein the asphalt is SBS modified asphalt, and the snow-melting and ice-inhibiting raw materials comprise biochemical humic acid, pyroligneous liquor, a starch-acrylic acid grafted copolymer, chloroprene emulsion, epoxy resin, phthalimide, isopropyl benzoate, polyester acrylate, beta-hydroxybutyraldehyde, thiourea, sorbitan fatty acid ester and the like. And after the snow-melting and ice-inhibiting material is mixed with the pavement material, the pavement performance of the pavement material can be improved, the loss of part of the snow-melting and ice-inhibiting material cannot be caused by rainwater scouring and vehicle abrasion, and a long-term effect can be achieved.
Owner:重庆市市政设计研究院有限公司
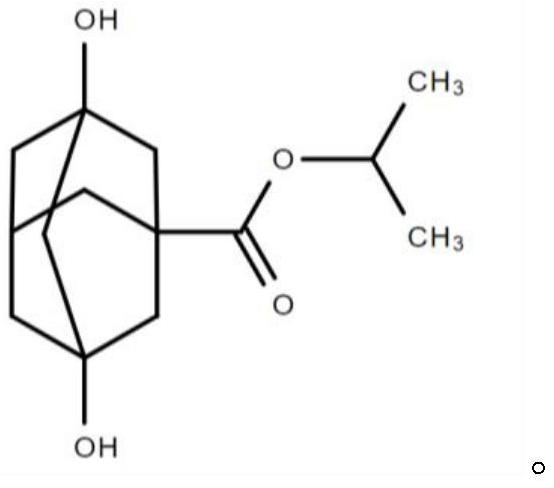
Battery foil rolling additive and preparation method and application thereof
ActiveCN113444559AImprove surface qualityIncrease surface dyne valueAdditivesAl powderIsopropyl formate
The invention provides a battery foil rolling additive as well as a preparation method and application thereof. The battery foil rolling additive comprises the following components in percentage by mass: 10-20% of fatty acid ester, 15-30% of fatty alcohol, 45-65% of an adamantane compound and 0.1-5% of an extreme pressure additive, wherein the mass percentage content of dihydroxy adamantane isopropyl formate in the adamantane compound is 50 to 100 percent. According to the battery foil rolling additive, through matching of the components, especially the use of the adamantane compound with a specific molecular structure, the battery foil rolling additive has excellent lubricating performance, the surface quality of a rolled battery foil can be improved, the surface dyne value of the battery foil is increased, and the cost of a corona process is reduced; and meanwhile, the use of the battery foil rolling additive is beneficial to improving the dispersing performance and filtering efficiency of aluminum powder and improving the cleaning performance of a rolling mill.
Owner:QUAKER CHEM CHINA
Aspartame production process
The invention discloses the technical field of an aspartame production process, and particularly relates to an aspartame production process which comprises the following steps: adding formic acid and magnesium oxide into a reaction kettle, stirring to completely dissolve magnesium oxide in formic acid, then adding acetic anhydride, isopropanol, L-phenylalanine and ethyl acetate into the reaction kettle, stirring at normal temperature, and when a reaction solution is viscous, stopping stirring to obtain an aspartame solution; acetic acid and the like are added, isopropyl alcohol is added into reactants to react with formic acid to generate isopropyl formate, and formic acid is removed, so that smooth proceeding of subsequent condensation reaction is guaranteed, and the yield of the final product aspartame is effectively improved; after the condensation reaction is finished, the solvent acetic acid is volatilized in advance in a centrifugal manner, so that most acetic acid is removed, the acetic acid and ester are removed by adding hydrochloric acid and methanol into the reaction kettle in a reduced pressure distillation manner, the methanol, residual acetic acid and hydrolyzed formic acid generate ester, and the ester is removed through distillation. And the high purity of the final product aspartame is ensured through multiple removal processes of the solvent.
Owner:JIANGSU HAN KUANG BIOLOGICAL ENG
Aldehyde oxidation processes
ActiveUS20160326083A1Reduce formationOrganic compound preparationPreparation by aldehyde oxidation-reductionStatic mixerIsopropyl formate
The oxidation of isobutyraldehyde produces isobutyric acid and byproducts, such as isopropyl formate. A process of reducing the isopropyl formate byproduct and other byproducts in the oxidation of isobutyraldehyde is described. The process uses a carbonyl compound, such as acetone, to reduce byproduct levels in the resulting product. Process for use of static mixers in oxidation reactions of aldehydes are also provided.
Owner:EASTMAN CHEM CO
Preparation method of sodium salt of pravastatin
ActiveCN101580466AReduce usageLow facility requirementsOrganic compound preparationMetabolism disorderIsopropyl formateSodium salt
The invention discloses a preparation method of sodium salt of pravastatin, which comprises the following steps: adding inorganic alkaline or organic alkaline containing sodium ions as positive ions into organic solution containing the pravastatin until pH is more than 7 and obtaining sodium pravastatin; the organic solution containing the pravastatin comprises the pravastatin and first solvent, wherein the first solvent is selected from one or a plurality of methyl formate, n-propyl formate, isopropyl formate, n-butyl formate, methyl acetate, ethyl acetate, n-propyl acetate, isopropyl acetate, n-butyl acetate, sec-butyl acetate, isobutyl acetate and tert-butyl acetate.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Synthesis method of diisopropyl azodicarboxylate
InactiveCN104478758ALow priceMild reaction conditionsOrganic chemistryDiisopropyl azodicarboxylateFiltration
The invention discloses a synthesis method of diisopropyl azodicarboxylate. The synthesis method comprises the following steps: (1) adding alkali and a solvent under protection of inert gases, sequentially adding carbazic acid isopropyl ester and diethyl carbonate, carrying out heating reaction at 100-180 DEGC for 2-6 hours, adjusting the pH value of a solution to 4.5-7.5 with acid, and carrying out suction filtration, so as to obtain hydrogenated diisopropyl azodiformate; and (2) adding 40%-50% of sulfuric acid solution to the hydrogenated diisopropyl azodiformate at -20 DEG C to 25 DEG C, dissolving, adding a catalyst, controlling the reaction temperature to -15 DEG C to -5 DEG C, dropwise adding 10%-15% of hydrogen peroxide until no heat is emitted, further reacting for 5-10 hours after dropwise adding is ended, quenching, extracting, washing to be neutral, drying, filtering, and concentrating to obtain the diisopropyl azodiformate. The synthesis method disclosed by the invention is clean and environmental friendly in raw material, mild in reaction condition, and few in byproducts; and the product is high in yield and beneficial to industrialized production.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Reduction of ester formation in isobutyraldehyde oxidation
ActiveUS9227903B2Reduce formationOrganic compound preparationCarboxylic preparation by ozone oxidationProduction rateIsopropyl formate
The oxidation of isobutyraldehyde produces isobutyric acid and byproducts, such as isopropyl formate. A method of reducing the isopropyl formate byproduct in the oxidation of isobutyraldehyde is described. The method uses a co-solvent, such as acetone, to the isobutyraldehyde feed to increase both the selectivity of the reaction to isobutyric acid and the production rate of isobutyric acid so that the isopropyl formate byproduct is significantly reduced.
Owner:EASTMAN CHEM CO
Aldehyde oxidation processes
ActiveUS20150166451A1Reduce formationOrganic compound preparationPreparation by aldehyde oxidation-reductionStatic mixerIsopropyl formate
The oxidation of isobutyraldehyde produces isobutyric acid and byproducts, such as isopropyl formate. A process of reducing the isopropyl formate byproduct and other byproducts in the oxidation of isobutyraldehyde is described. The process uses a carbonyl compound, such as acetone, to reduce byproduct levels in the resulting product. Process for use of static mixers in oxidation reactions of aldehydes are also provided.
Owner:EASTMAN CHEM CO
Application of composition in preparation of medicine for treating liver insulin resistance
InactiveCN104274448AEffective treatmentOrganic active ingredientsMetabolism disorderIsopropyl formate1H-tetrazole
The invention discloses application of a composition in preparation of a medicine for treating liver insulin resistance. The composition contains a therapeutically-effective amount of 1'-(1H-indazol-5-carbonyl)-1-isopropyl-4,6-dihydrospiro[indazol-5,4'-piperidine]-7(1H)-one and a therapeutically-effective amount of 4-[5-cyano-4-({2-fluoro-4-[1-(2-hydroxyethyl)-1H-tetrazol-5-yl]phenoxy}methyl)-1H-pyrazol-1-yl]piperidin-1-isopropyl formate which have the mass ratio of (3: 1) to (1: 3). Proved by experiments, by using the two kinds of drugs in a combined manner, the liver insulin resistance can be effectively treated, and concomitant symptoms, such as hypertension and diabetes, can be treated while the liver insulin resistance is treated.
Owner:WUXI XINDA MEDICAL DEVICE
A kind of sbs graft type clean odor mattress spray glue and preparation method thereof
ActiveCN107177332BIncrease forceReduce the numberMacromolecular adhesive additivesGraft polymer adhesivesElastomerPolymer science
The invention discloses a SBS grafted type deodorizing mattress spray glue and a preparation method thereof, comprising the following components in parts by weight: 10-15 parts of methyl isobutyl ketone; 5-10 parts of isopropyl formate; aromatics 10-20 parts of environmentally friendly solvent oil; 15-25 parts of methylcyclohexane; 10-18 parts of SBS elastomer; 0.1-0.5 parts of BPO; 5-15 parts of MMA; 0-8 parts of BA; 1-3 parts of terminator; 10-15 parts of hydrogenated petroleum resin; 12-18 parts of hydrogenated rosin modified resin. The SBS grafted odor-free mattress spray glue of the present invention is colorless and transparent, has strong adhesive force, is resistant to yellowing, chalking, high temperature, oil, plasticizer, non-toxic, odorless and residue-free. It is an ideal glue spraying choice for mattress manufacturers. During the spraying process, the odor is small, and the film has no residual solvent and resin odor. The film is white and transparent. After sizing, it will not pollute light-colored mattress fabrics.
Owner:佛山市东方树新材料科技有限公司
Method for determining residual content of 4-methylpiperazine-1-formate genotoxic impurities in zopiclone
InactiveCN113552241AHigh penetration rateReduce use costComponent separationIsopropyl formateVolumetric flask
The invention discloses a method for determining the residual contents of 4-methyl piperazine-1-methyl formate, 4-methyl piperazine-1-ethyl formate and 4-methyl piperazine-1-isopropyl formate in zopiclone. The method comprises the following steps: preparing a contrast solution: precisely weighing a proper amount of methyl 4-methylpiperazine-1-formate, a proper amount of ethyl 4-methylpiperazine-1-formate and a proper amount of isopropyl 4-methylpiperazine-1-formate, putting the weighed materials into a volumetric flask, dissolving the weighed materials with acetonitrile, diluting the dissolved materials, and respectively preparing 125ng / mL limit concentration solutions; preparing a test sample solution: precisely weighing a proper amount of zopiclone test sample, placing the zopiclone test sample in a volumetric flask, dissolving and diluting the zopiclone test sample with acetonitrile, and preparing the zopiclone test sample into a 1mg / mL solution; and sample determination: respectively taking the test solution and the reference solution, directly injecting the test solution and the reference solution into a GCMS, recording a chromatogram, and calculating by peak areas according to an external standard method to obtain the contents of the 4-methylpiperazine-1-methyl formate, the 4-methylpiperazine-1-ethyl formate and the 4-methylpiperazine-1-isopropyl formate in the test solution.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Application of composition in preparation of medicine for treating type 2 diabetes
InactiveCN104274445AEffective treatmentOrganic active ingredientsMetabolism disorderIsopropyl formate1H-tetrazole
The invention discloses application of a composition in preparation of a medicine for treating type 2 diabetes. The composition contains a therapeutically-effective amount of 1'-(1H-indazol-5-carbonyl)-1-isopropyl-4,6-dihydrospiro[indazol-5,4'-piperidine]-7(1H)-one and a therapeutically-effective amount of 4-[5-cyano-4-({2-fluoro-4-[1-(2-hydroxyethyl)-1H-tetrazol-5-yl]phenoxy}methyl)-1H-pyrazol-1-yl]piperidin-1-isopropyl formate which have the mass ratio of (3: 1) to (1: 3). The composition of the medicine can be used for effectively treating the type 2 diabetes.
Owner:WUXI XINDA MEDICAL DEVICE
A kind of detection method of formic acid in ant products
ActiveCN111595984BImprove accuracyImprove conversion rateComponent separationPtru catalystReaction rate
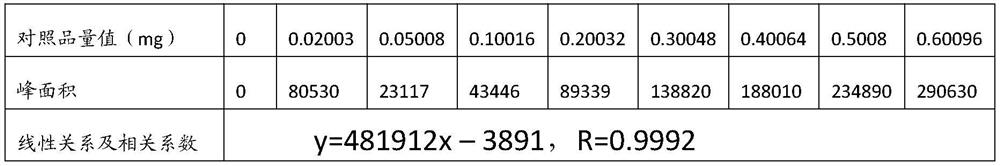
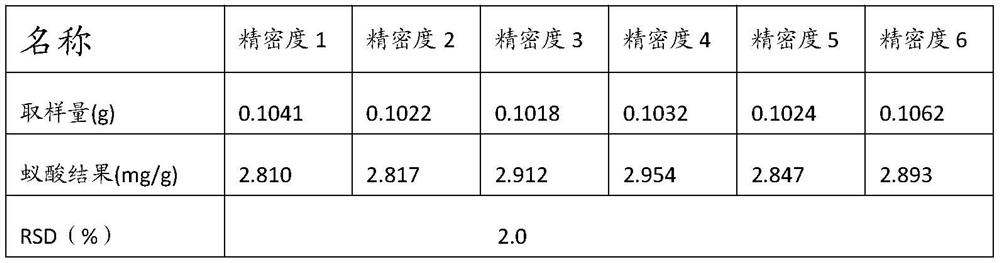
The invention relates to the technical field of formic acid detection, and provides a detection method for formic acid in ant products. By selecting isopropanol as an extractant and a reactant to carry out an esterification reaction, the reaction is sufficient, and there is almost no interference between substances. Therefore, the accuracy of the test method is improved; at the same time, concentrated sulfuric acid and p-toluenesulfonic acid are used as the catalyst for the esterification reaction, which avoids the occurrence of side effects such as sulfonation, carbonization or polymerization due to the violent reaction when the concentrated sulfuric acid is used as the catalyst alone. The reaction occurs; the problem of poor selectivity of isopropyl formate and too slow reaction rate when single selection of p-toluenesulfonic acid is solved, through the coordinated use of the two, the reaction rate is moderate, and the transformation rate of the product isopropyl formate is simultaneously improved, This further improves the accuracy of the test results.
Owner:SHANXI PROVINCE FOOD & DRUG INSPECTION INST
Pharmaceutical composition for treating liver insulin resistance
InactiveCN104274450AEffective treatmentOrganic active ingredientsMetabolism disorderIsopropyl formate1H-tetrazole
The invention discloses a pharmaceutical composition for treating liver insulin resistance. The pharmaceutical composition contains a therapeutically-effective amount of 1'-(1H-indazol-5-carbonyl)-1-isopropyl-4,6-dihydrospiro[indazol-5,4'-piperidine]-7(1H)-one and a therapeutically-effective amount of 4-[5-cyano-4-({2-fluoro-4-[1-(2-hydroxyethyl)-1H-tetrazol-5-yl]phenoxy}methyl)-1H-pyrazol-1-yl]piperidin-1-isopropyl formate which have the mass ratio of (3: 1) to (1: 3). Proved by experiments, by using the two kinds of drugs in a combined manner, the liver insulin resistance can be effectively treated, and concomitant symptoms, such as hypertension and diabetes, can be treated while the liver insulin resistance is treated.
Owner:WUXI XINDA MEDICAL DEVICE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com