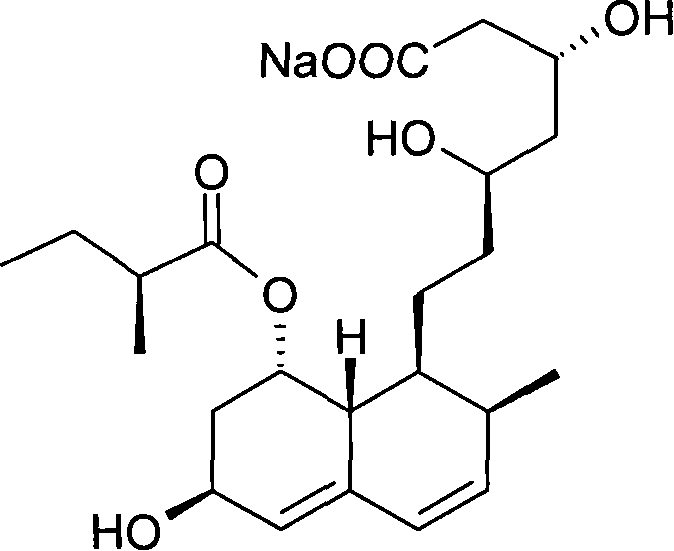
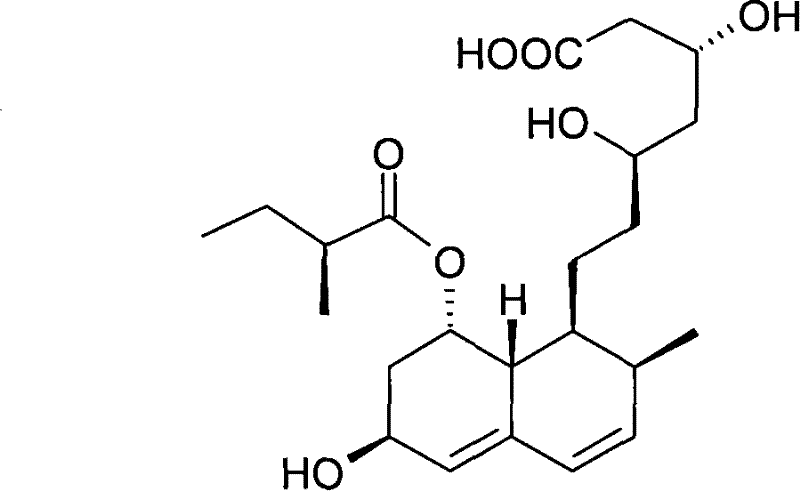
Preparation method of sodium salt of pravastatin
A technology of sodium naphthalene and naphthalene, which is applied in the preparation of organic compounds, the preparation of carboxylate, chemical instruments and methods, etc. safety and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] In another preference of the present invention, the preparation method of described naveladine sodium comprises the following steps:
[0044](a) Naveladidine salt is transferred to the first solvent by extraction to form an organic solution containing naveladine; the first solvent is selected from one or more of the following groups: methyl formate, formic acid n-propyl, isopropyl formate, n-butyl formate, methyl acetate, ethyl acetate, n-propyl acetate, isopropyl acetate, n-butyl acetate, sec-butyl acetate, isobutyl acetate, tert-butyl acetate ester;
[0045] (b) adding an inorganic or organic base whose cation is a sodium ion to the organic solution obtained in step (a), to obtain a precipitate;
[0046] (c) Repeat the steps from (a) to (b) for the precipitate obtained in step (b) for 1-5 times to obtain naveladine sodium; preferably repeat 1-3 times.
[0047] In the present invention, the organic solution containing naveladidine can be obtained using methods famili...
Embodiment 1
[0071] 35 grams of naveladidine (purchased from Shanghai Tianwei Biopharmaceutical Co., Ltd.) was completely dissolved in 750 mL of ethyl acetate at 20 ° C, and then a methanol solution containing 1M NaOH was added thereto until the pH was above 7. 80 mL of methanol solution of 1M NaOH was collected by filtration and dried in vacuo to obtain 31 grams of white crystalline powder of naveladidine sodium.
[0072] The specific rotation of naveledadine sodium is +157°, the purity is 99.95%, and the content of the structural analogue 3α-naveledadine sodium is 0.03%.
Embodiment 2
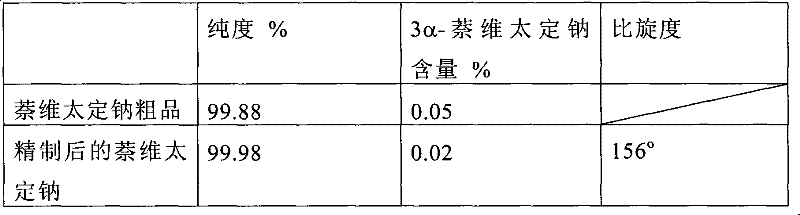
[0074] 350 grams of naveladine sodium crude product was completely dissolved in 2L of water at 20°C, then 4L of ethyl acetate was added thereto, stirred, and washed with 0.5M H 2 SO 4 Adjust the pH below 4, statically discard the water phase, and then add an ethanol solution containing 1M NaOH to it until the pH is above 800mL, add a total of 800mL of an ethanol solution containing 1M NaOH, collect the precipitate by filtration, and dry it in vacuum to obtain the refined naphthalene Taiding sodium white crystalline powder 330 grams.
[0075] Its assay result and the comparative data with naveladine sodium crude product purity are as follows:
[0076]
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com