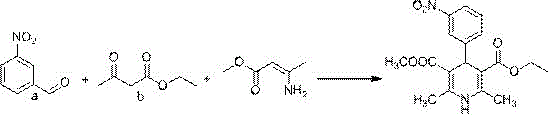
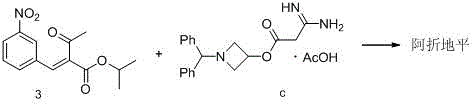
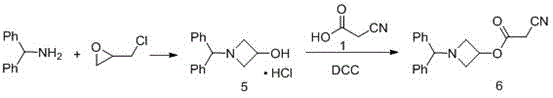
Preparation method of azelnidipine
A technology for azeldipine and a compound, which is applied in the field of preparation of azeldipine, can solve the problems of increased space barriers, cumbersome operations, large changes in chemical properties, etc., and achieves the advantages of reducing impurities, improving product quality, and meeting the requirements of medicinal standards. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The first step Pinner reaction
[0036] Add 750 g of dichloromethane, 65.65 g (1.43 mol) of absolute ethanol and 120 g (1.42 mol) of nitrile acetic acid (compound 1) into the reaction flask in sequence, mix and stir, and cool down to -10°C~0°C. Pass dry hydrogen chloride gas until saturated, and keep the reaction for 8~12h to the end. The solvent was recovered under reduced pressure at room temperature, 600 g of dichloromethane was added to the residue, mixed and stirred, and the temperature was lowered to -5°C~0°C, dry ammonia gas was introduced until saturation, and the reaction was kept for 3~5h to the end. The solvent was recovered under reduced pressure at room temperature to obtain 137.4 g of amidine acetic acid (compound 2). The yield in this step is 94.8%, and the HPLC purity is 99.6%.
[0037] Second step Hantzsch reaction
[0038] Add 520g of isopropanol, 106g (1.04mol) of amidine acetic acid (compound 2) and 288.6g (1.06mol) of compound 3 to the reaction f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com