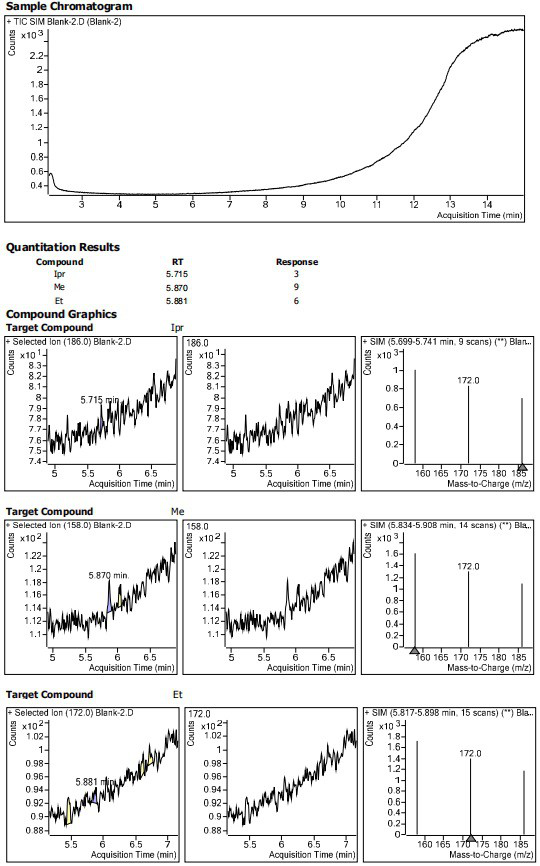
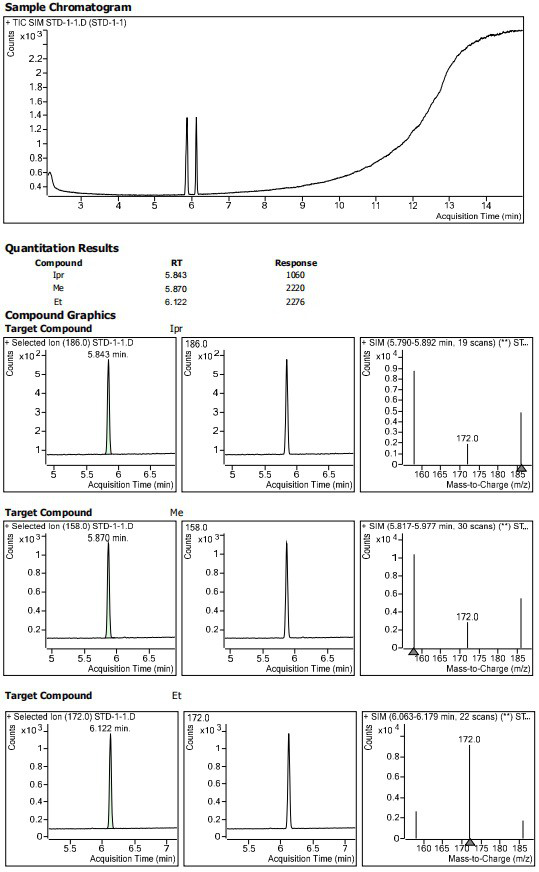
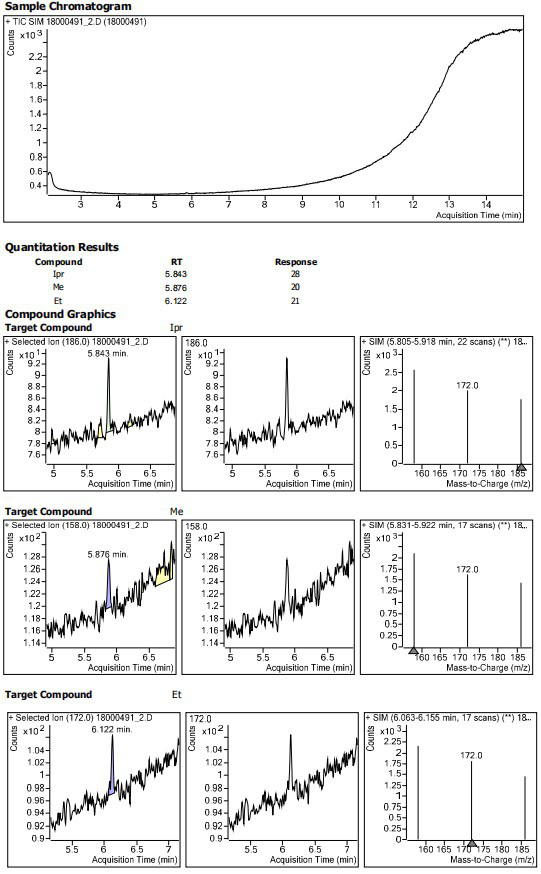
Method for determining residual content of 4-methylpiperazine-1-formate genotoxic impurities in zopiclone
A technology of methylpiperazine and zopiclone, which is applied in the field of medicine, can solve problems such as lack of standards, potential safety hazards, and inability to evaluate the quality of medicines, achieving high accuracy, high equipment penetration rate, and good separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0070] Blank solvent: Acetonitrile
[0071] Preparation of reference solution
[0072] Weigh 0.01024 g of methyl 4-methylpiperazine-1-carboxylate into a 10 mL brown volumetric flask, add diluent to dissolve and dilute to the mark to obtain methyl 4-methylpiperazine-1-carboxylate stock solution I.
[0073] Weigh 0.00998 g of ethyl 4-methylpiperazine-1-carboxylate into a 10 mL brown volumetric flask, add diluent to dissolve and dilute to the mark to obtain ethyl 4-methylpiperazine-1-carboxylate stock solution I.
[0074] Weigh 0.00995 g of isopropyl 4-methylpiperazine-1-carboxylate into a 10 mL brown volumetric flask, add diluent to dissolve and dilute to the mark to obtain isopropyl 4-methylpiperazine-1-carboxylate stock solution I.
[0075] Precisely pipette 125 μL of the above 4-methylpiperazine-1-carboxylic acid methyl ester stock solution Ⅰ, 4-methylpiperazine-1-carboxylic acid ethyl ester stock solution Ⅰ 125 μL, 4-methylpiperazine-1-carboxylic acid isopropyl ester 125 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com