In-vitro in-vivo drug release characteristics of various drug pills in compound anti-tuberculosis preparation and application thereof
A drug release and preparation technology, which is applied to the in vivo and in vitro drug release characteristics and application fields of each drug pellet in the compound anti-tuberculosis preparation, and can solve the problems of inability to obtain in vivo absorption pellets and different drug release characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
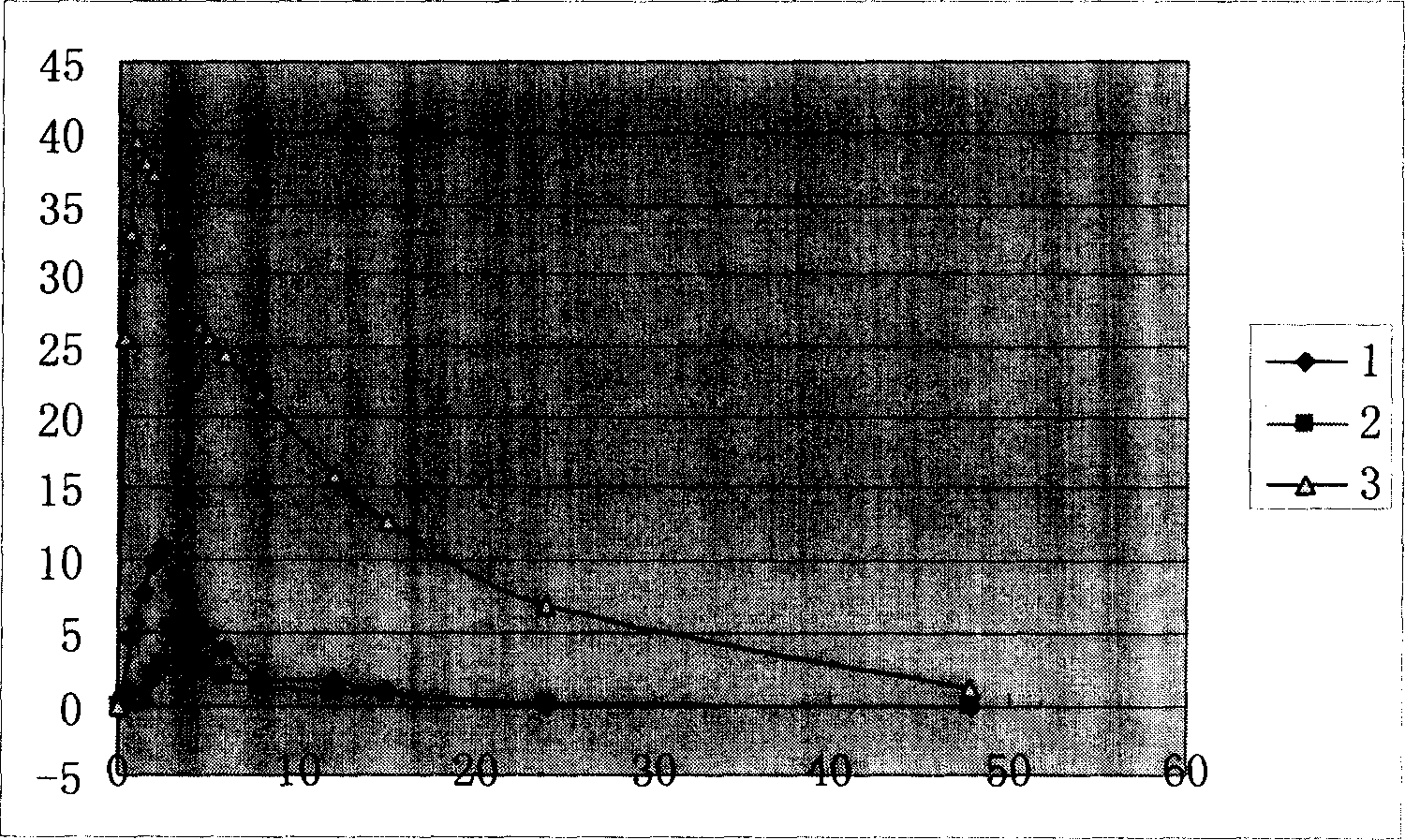
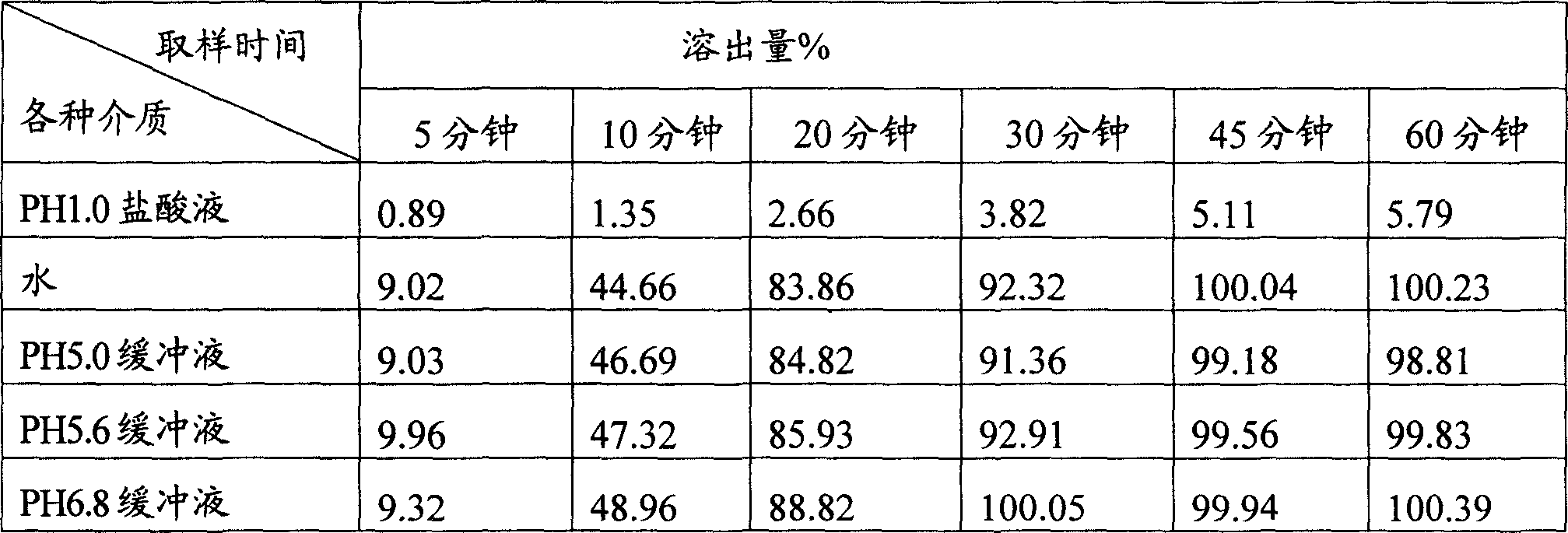
[0027] Example 1 The in vitro release characteristics of rifampicin enteric-coated pellets:
[0028] 1. Experimental purpose: To determine the dissolution curve of enteric-coated rifampicin pellets in hydrochloric acid solution (9→1000), water, pH5.0, pH5.6, pH6.8 phosphate buffer.
[0029] 2. Experimental basis: method 2 of the second method under the second appendix of the 2005 edition of the Chinese Pharmacopoeia "Determination of release rate". Get this product (equivalent to rifampicin 50mg) respectively according to the first method device of dissolution assay, respectively with water, hydrochloric acid solution (9 → 1000), pH5.0 phosphate buffer saline, pH5.0 phosphate buffer saline, pH 6.8 phosphate buffer 900ml as the solvent, rotate at 50 rpm, operate according to the law, take 10ml of the solution at 5 minutes, 10 minutes, 20 minutes, 30 minutes, 45 minutes, and 60 minutes, filter, and replenish the same volume For the solvent, take 4ml of the continued filtrate an...
Embodiment 2
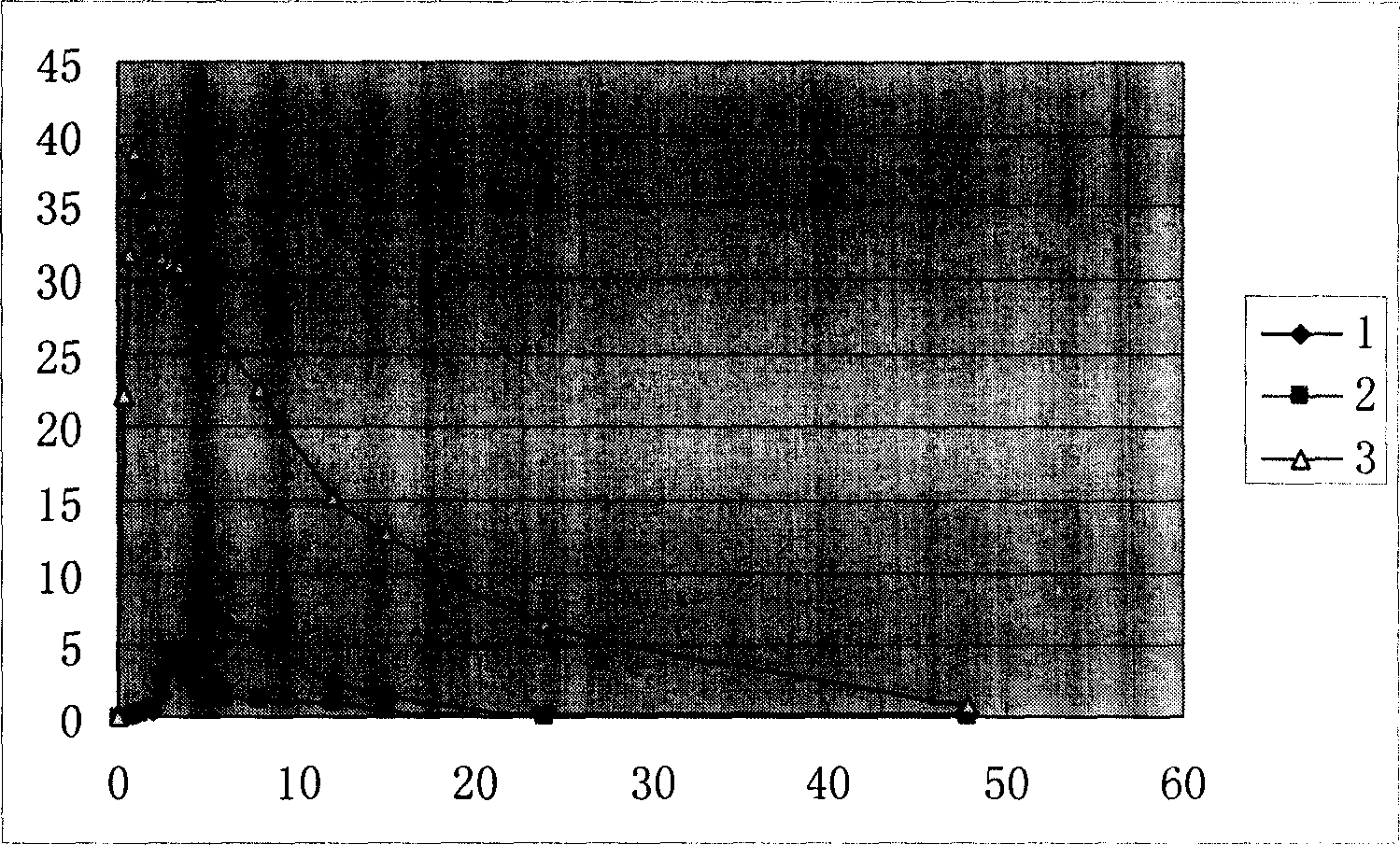
[0032] Example 2 In vitro release characteristics of isoniazid enteric-coated pellets:
[0033] 1. Experimental purpose: To determine the dissolution curve of enteric-coated isoniazid pellets in hydrochloric acid solution (9→1000), water, pH6.8 phosphate buffer, and pH5.0 phosphate buffer.
[0034] 2. Experimental basis: method 2 of the second method under the second appendix of the 2005 edition of the Chinese Pharmacopoeia "Determination of release rate". Take this product (equivalent to isoniazid 150mg) according to the first method of dissolution assay, respectively, with hydrochloric acid solution (9 → 1000), water, pH5.0 phosphate buffer, pH6.8 phosphate buffer 900ml As a solvent, the rotation speed is 50 rpm, operate according to the law, take 5ml of the solution at 10 minutes, 20 minutes, 30 minutes, 45 minutes, and 60 minutes, filter, and replenish the same volume of solvent at the same time, take 1ml of the filtrate and put it in a 10ml volume Dilute to the mark with...
Embodiment 3
[0037] Example 3 The in vitro drug release characteristics of rifampicin stomach-dissolved pellets
[0038] 1. Experimental purpose: To determine the dissolution curve of rifampicin Weirong pellets in hydrochloric acid solution (9→1000).
[0039] 2. Experimental basis: the second appendix of the Chinese Pharmacopoeia 2005 edition "dissolution assay", take this product (equivalent to rifampicin 75mg) according to the first method of dissolution assay, using 900ml of hydrochloric acid solution (9→1000) as solvent , using a speed of 50 rpm, operate according to the law; take 10ml of the solution at 5, 10, 15, 20, 30, and 45 minutes, filter, and replenish the same volume of solvent at the same time. Accurately measure 3ml of continued filtrate, put in a 10ml measuring bottle, dilute to the mark with hydrochloric acid solution (9→1000), and use it as the test solution; take rifampicin reference substance in addition, and dilute it with hydrochloric acid solution (9→1000) to form T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com