A medicine for treating osteoporosis
A technology for osteoporosis and medicine, application in medicine, medicine for treating osteoporosis, and the field of preparation of the above medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
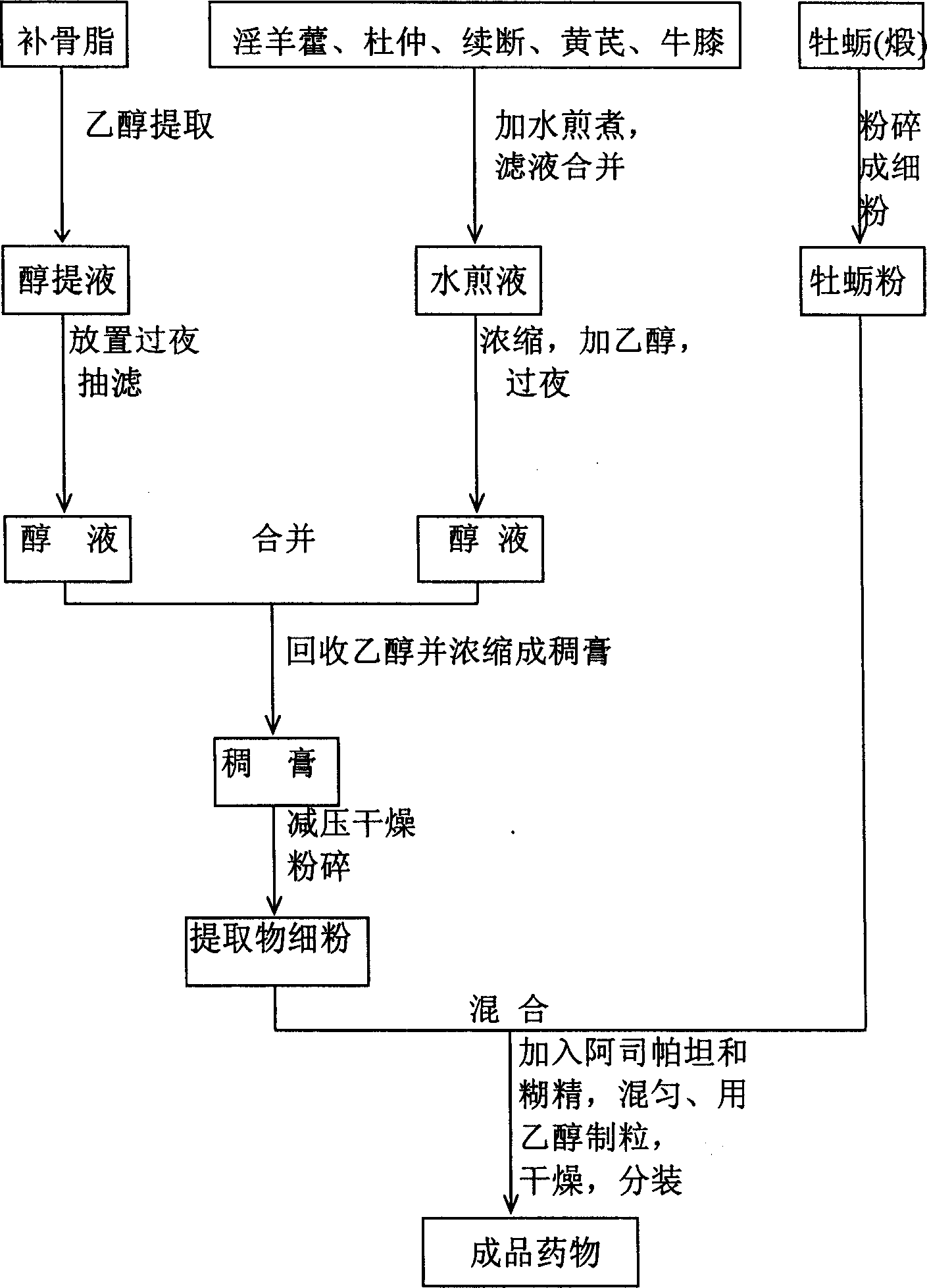
[0107] Four, preparation technology of the present invention
[0108] 1) Selection of process parameters:
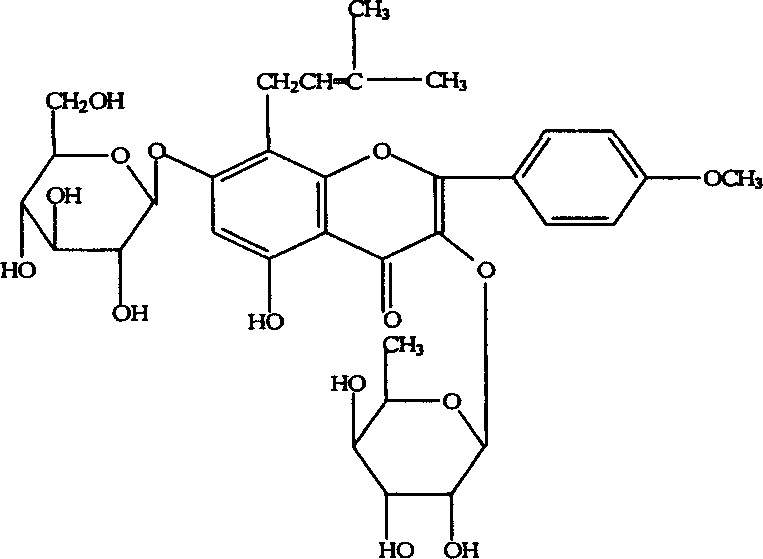
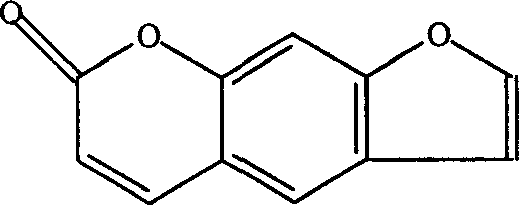
[0109] 1. Optimal selection of process parameters for alcohol extraction of psoralen
[0110] (1) Assay method of psoralen:
[0111] Octadecylsilane bonded silica gel is used as filler; methanol-water (50:60) is used as mobile phase; detection wavelength is 295nm; column temperature is 35°C.
[0112] Preparation of the test solution: Weigh 0.2 g of the dry extract, add 10 ml of methanol to dissolve, filter, take 5 ml of the subsequent filtrate, and filter with a microporous membrane (0.5um) to obtain final product.
[0113] Preparation of reference substance solution: Weigh an appropriate amount of psoralen reference substance dried at 105°C to constant weight, add mobile phase to make a solution containing 0.125 mg of psoralen per 1 ml, as the reference substance solution.
[0114] Determination method: Draw 3ul of the reference substance solution and 1ul of the test...
Embodiment 1
[0173] Embodiment one (the medicine prepared by the present invention is called Xianniu Jiangu Granule in the following examples)
[0174] Oyster (calcined) 50g is crushed into fine powder. 300 g of psoraleae was added with 4 times the amount of 70% ethanol to extract three times, each time for 2 hours, and the decoction was combined and filtered. Add 500g of Epimedium, 400g of Eucommia, 300g of Dipsacus, 300g of Astragalus, and 300g of Achyranthes bidentata, add water and decoct three times, each time for 1 hour, add water 14 times the amount of medicinal materials, combine the decoction, filter, and concentrate the filtrate to relative density 1.10-1.15 (50°C), add ethanol to make the alcohol concentration reach 70%, let stand overnight, filter, combine the filtrate with the above-mentioned bakuchiol alcohol extract, recover the ethanol and concentrate to a relative density of 1.25-1.30 (50°C) , dried under reduced pressure, crushed into fine powder, and mixed with oyster (...
Embodiment 2
[0177] Drug acute toxicity test (maximum dosage test)
[0178] The purpose of this experiment is to observe the acute toxic reaction and death caused by oral administration of Xianniu Jiangu Granules in mice.
[0179] According to the relevant regulations on the research of new traditional Chinese medicines, the acute toxicity experiment in mice was observed with Xianniu Jiangu Granules. LD cannot be detected 50 , so the determination of the maximum dose was carried out. Mice were administered intragastrically, and the cumulative dose was 102.8g of crude drug / kg, which was 112 times that of clinical medication. No adverse reaction or death was found in the animals.
[0180] experiment material
[0181] Xianniu Jiangu Granules, 3.85g crude drug / ml.
[0182]40 Kunming mice, half male and half male, weighing 19.80±1.34 g (n=40), were provided by the Experimental Animal Center of the Chinese Academy of Sciences. Certificate No. (1999) No. 036.
[0183] Test method: Animals...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com