Antybody therapy
A technology of antibody and therapeutic agent, applied in the field of antibody therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0132] CLAIMS 1. A composition comprising at least one anti-CEA monoclonal antibody (MAb) or fragment thereof and at least one therapeutic agent. The composition of embodiment 1, wherein the anti-CEA MAb is a class I, class II or class III anti-CEA MAb, and when the MAb is a class I or class II MAb and reacts with granulocytes, the MAb is a MAb Unit price form.
[0133]2. The composition of embodiment 1, wherein the anti-CEA MAb or fragment thereof is humanized, and wherein the humanized MAb substantially retains the anti-CEA binding specificity of the murine anti-CEA MAb.
[0134] 3. The composition of embodiment 1, wherein said anti-CEA MAb or fragment thereof is a chimeric MAb, and wherein said chimeric MAb substantially retains the anti-CEA binding specificity of a murine anti-CEA MAb.
[0135] 4. The composition of embodiment 1, wherein said anti-CEA MAb or fragment thereof is an intact human MAb, and wherein said human MAb substantially retains the anti-CEA binding spec...
Embodiment 1
[0254] Example 1: Materials and methods
[0255] Monoclonal Antibodies and Cell Lines
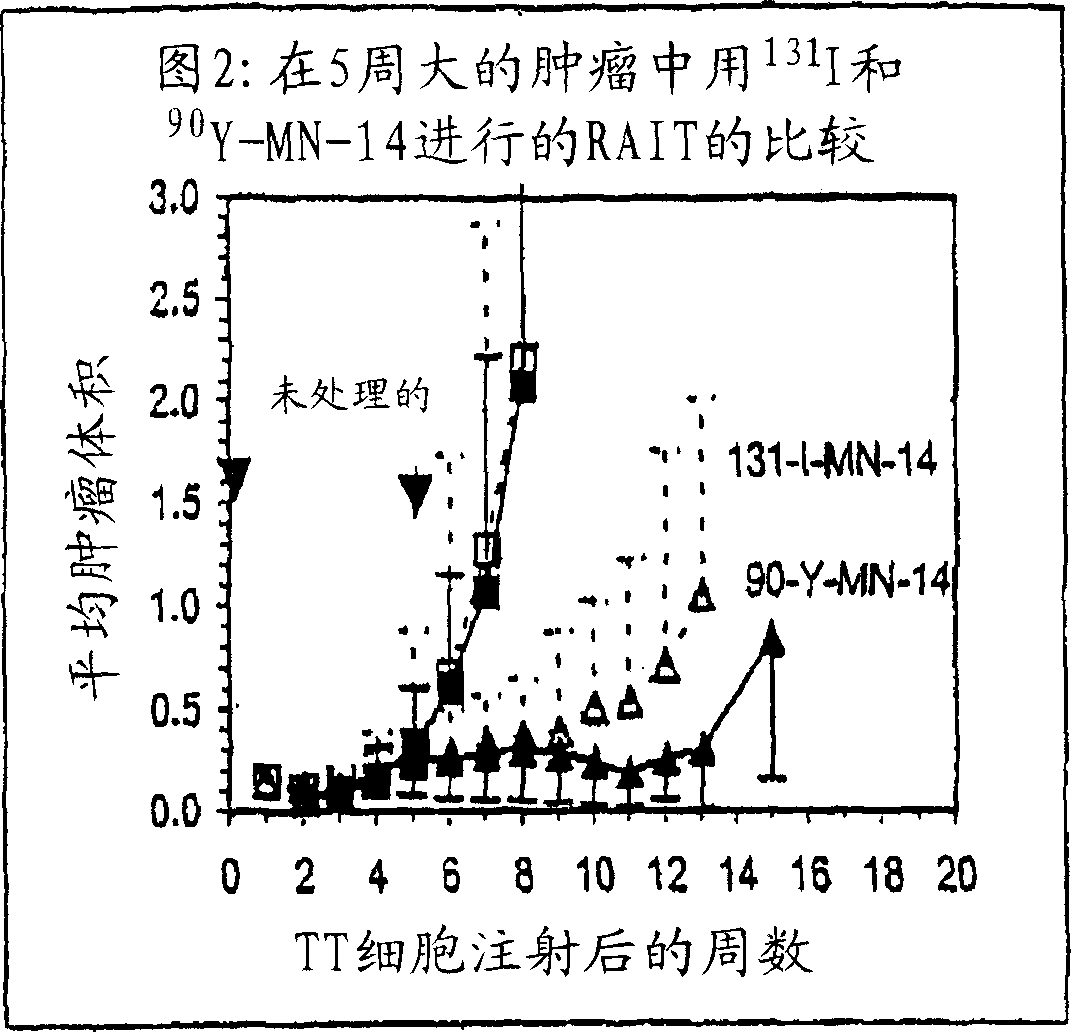
[0256] A human thyroid myeloid cell line, TT, was purchased from the American Type Culture Center. The cell monolayer was grown in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum, penicillin (100 U / ml), streptomycin (100 pg / ml) and L-glutamine (2mM). Cells were routinely passaged after detachment with trypsin, 0.2% EDTA.
[0257] MN-14 is a class III anti-CEA MAb reactive with CEA and unreactive with the normal cross-reactive antigens NCA and meconium antigen (Hansen et al., Cancer, 71:3478 (1993)). The construction and characterization of humanized versions of MN-14 and LL2, the anti-CD22 MAb used here as negative controls, has been presented previously. (Sharkey et al., Cancer Res., 55:5935s (1995); Leung et al., Mol. Immunol., 32:1416 (1995)). P3 x 63Ag8 (MOPC-21) is an unrelated mouse myeloma IgGl obtained from the American Type Culture Center (R...
Embodiment 2
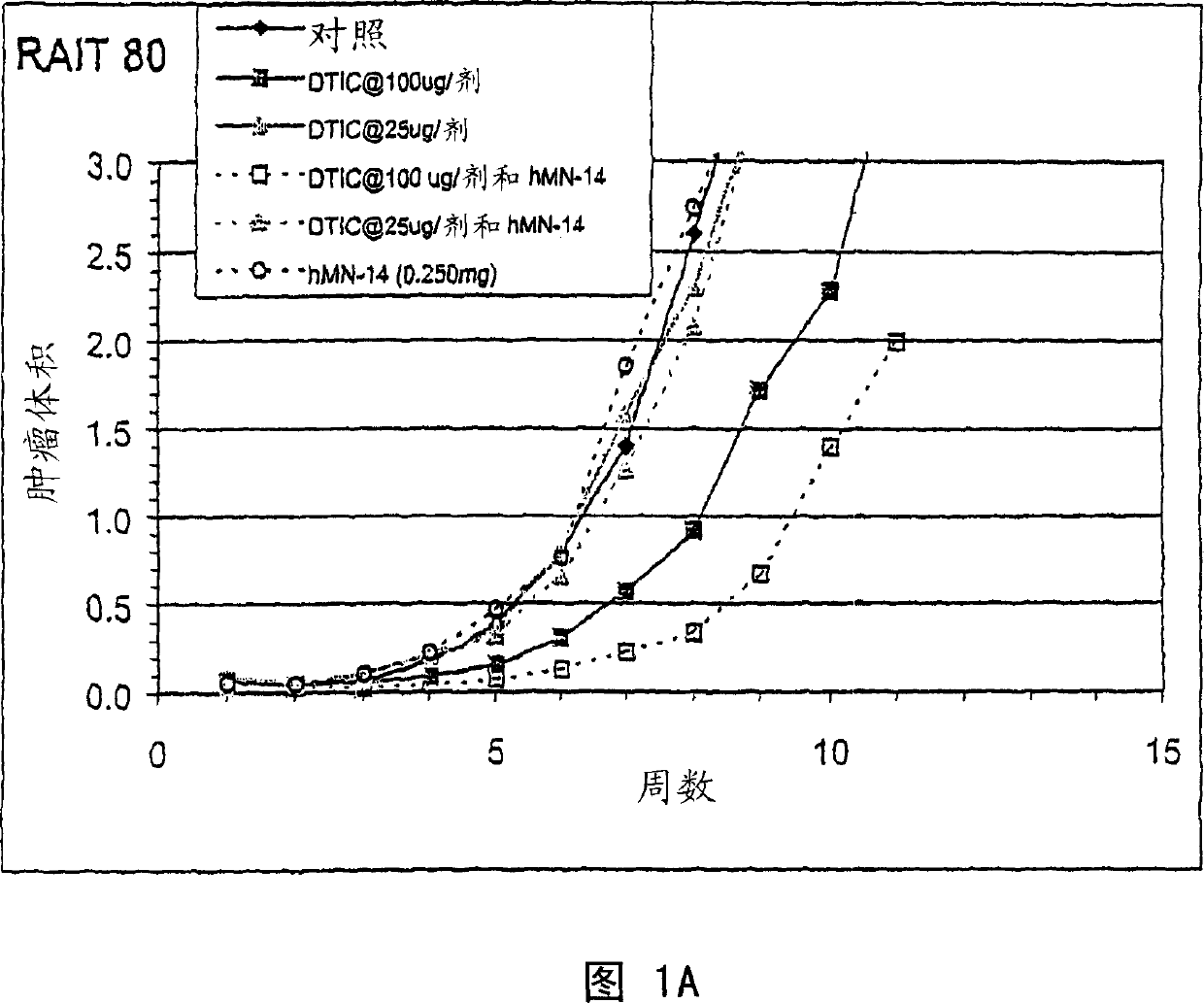
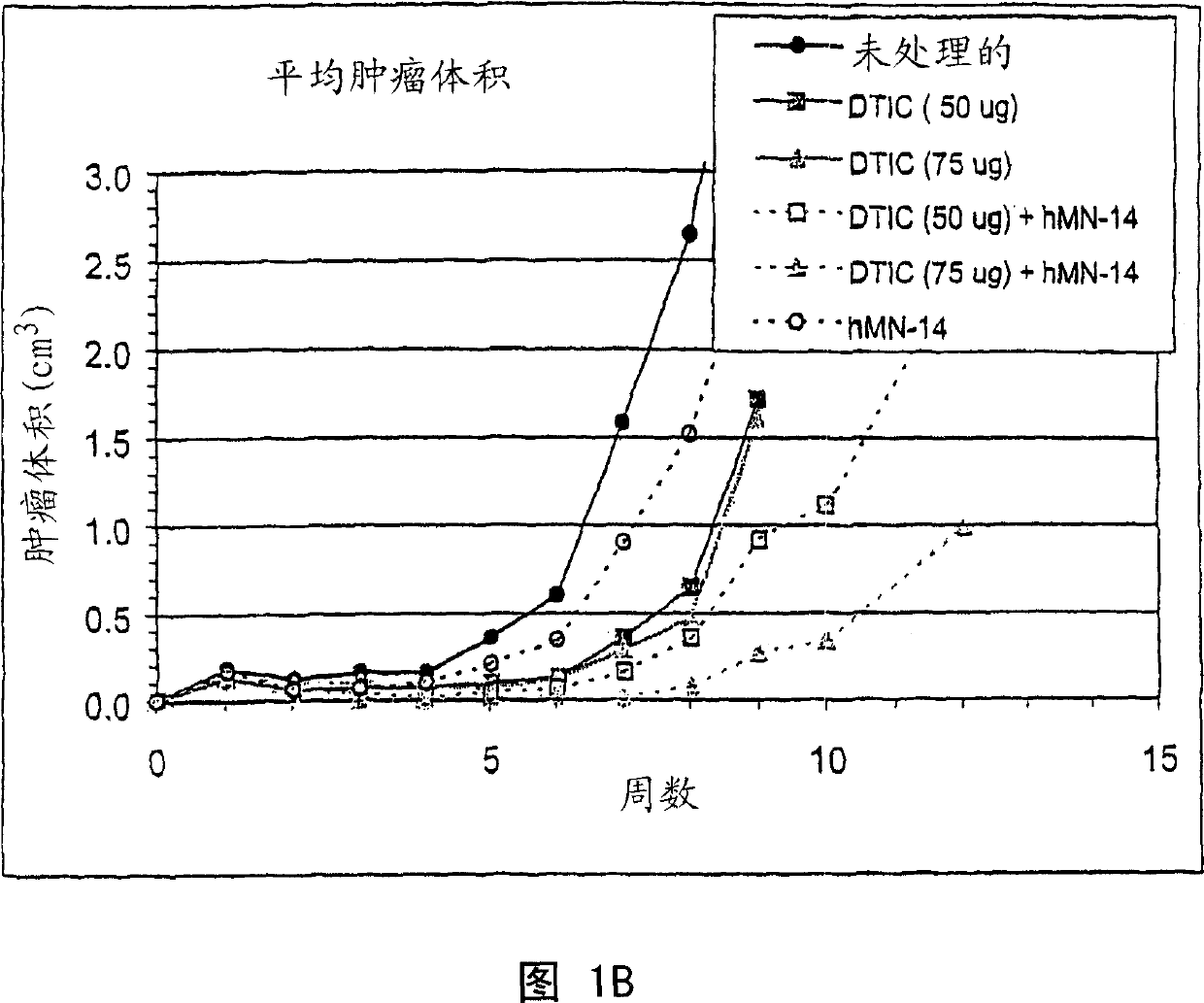
[0260] Example 2. Combination therapy of naked hMN-14 and DTIC delivered 2 days after injection of TT (human medullary thyroid) tumor cells
[0261]In the previous study, 100 μg and 25 μg doses of DTIC (days 2, 3 and 4) and 250 μg doses of hMN-14 given weekly on day 2 and thereafter in combination with TT were used 2 days after tumor implantation. Naked hMN-14 and dacarbazine were administered. A DTIC dose of 100 [mu]g combined with hMN-14 was more effective than either treatment alone (Fig. 1A). However, a dose of 100 μg of DTIC produced too strong a response, while a dose of 25 μg was ineffective. Surprisingly, the effects of MN-14 alone and DTIC alone were not additive. In other words, given the results of treatment with 250 μg hMN-14 alone and 100 μg DTIC alone, it would not have been predicted that the combination of 250 μg hMN-14 and 100 μg DTIC would have such a significant effect. See Figure 1A.
[0262] In this study, as in previous studies, treatment started 2 da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com