Medicine for treating gap and vomiting due to nuclear-radiation, radiotherapy and anti-motion sickness
A technique for nausea, vomiting, and motion sickness, applied in the digestive system, drug combination, aerosol delivery, etc., can solve unrealistic problems such as unfavorable preparation, use, transportation, and storage, and achieve effective anti-dizziness effects, overcome drowsiness, strong synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
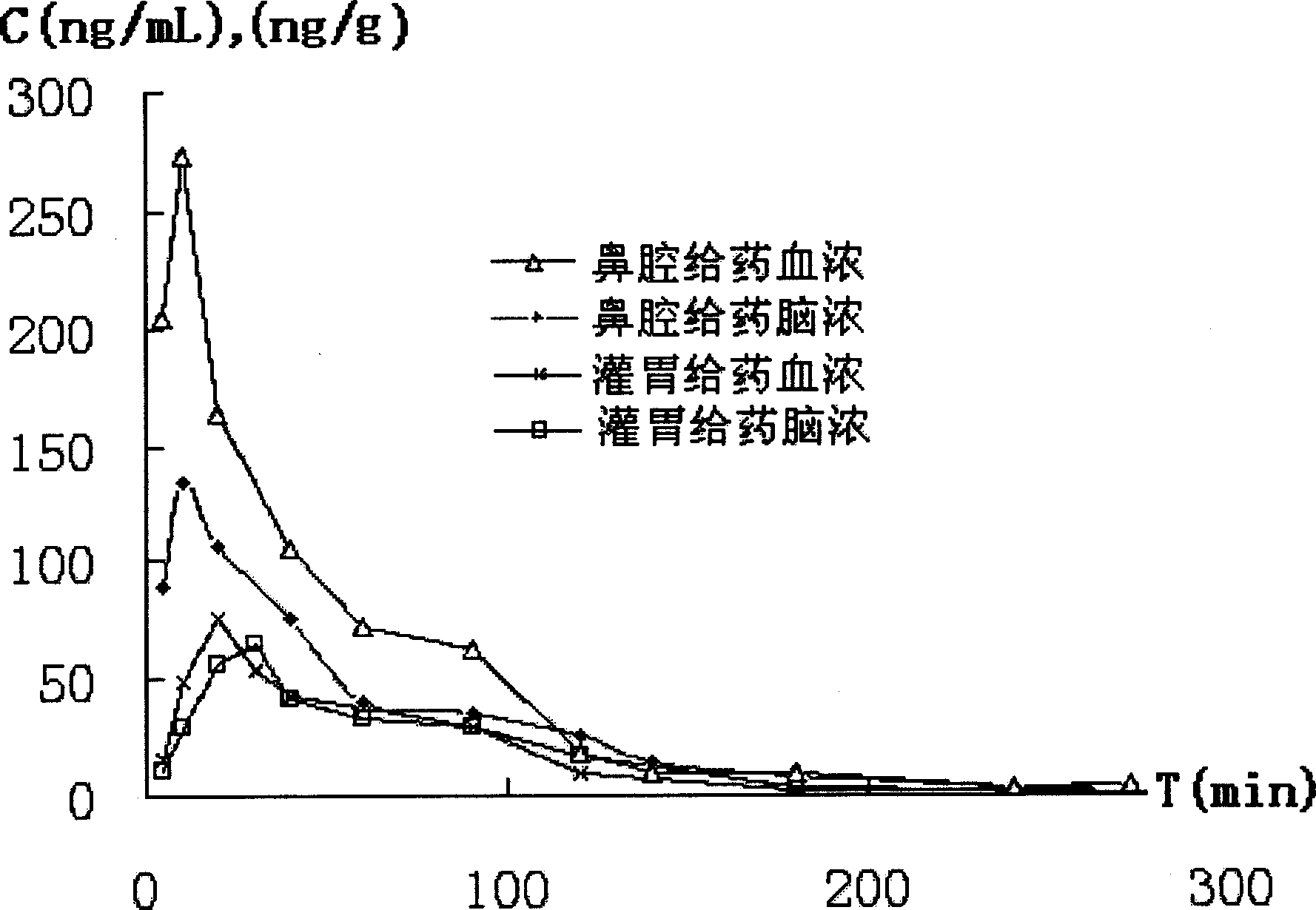
Image
Examples
Embodiment 1
[0018] Example 1: main drug: a mixture of ondansetron hydrochloride and scopolamine: 2g, auxiliary drugs: cyclodextrin: 7g, polyethylene glycol 400: 11g, white petrolatum: 6g, liquid paraffin: 6g, stearic acid: 4g, Cetyl alcohol: 4g, Span 60: 4g, appropriate amount of water to 100g.
[0019] Preparation method: Weigh white vaseline: 6g, liquid paraffin: 6g, stearic acid: 4g, cetyl alcohol: 4g and Span 60: 2g, mix well, and heat and stir to form a colorless and clear liquid as liquid A for later use; Then weigh the mixture of ondansetron hydrochloride and scopolamine: 2g, add it to water, then add cyclodextrin: 7g, stir to dissolve it into a colorless and clear solution, then add a part of polyethanol 400:11g and Span 60:2g After dissolving, it becomes liquid B; at this time, heat liquid A again, add liquid B while stirring to form a uniform emulsion, cool, stir to make the emulsion into a fine cream, and then pack it into the warehouse.
Embodiment 2
[0020] Example 2: main drug: ondansetron hydrochloride: 4g, auxiliary drug: cyclodextrin: 8g, polyethylene glycol 400: 9g, white petrolatum: 4g, liquid paraffin: 7g, stearic acid: 6g, stearyl alcohol: 6g, Tween 80: 6g, appropriate amount of water to 100g.
[0021] Preparation method: Weigh white vaseline: 4g, liquid paraffin: 7g, stearic acid: 6g, stearyl alcohol: 6g and Tween 80: 3g, mix well, and heat and stir to form a colorless and clear liquid as liquid A for later use; Then weigh ondansetron hydrochloride: 4g and add it to water, then add cyclodextrin: 8g, stir to make it dissolve into a colorless and clear solution, then add a part of polyethanol 400: 9g and Tween 80: 3g, and dissolve immediately It is liquid B; at this time, heat liquid A again, add liquid B while stirring to form a uniform emulsion, cool, stir to make the emulsion into a fine cream, and then pack it into the warehouse.
Embodiment 3
[0022] Example 3: main drug: scopolamine: 0.5g, auxiliary drug: cyclodextrin: 3g, polyethylene glycol 400: 14g, white petrolatum: 3g, liquid paraffin: 5g, stearic acid: 5g, stearyl alcohol: 7g, department Plate 60: 5g, appropriate amount of water to 100g.
[0023] Preparation method: Weigh white vaseline: 3g, liquid paraffin: 5g, stearic acid: 5g, cetyl alcohol: 7g and Span 60: 2g, mix evenly, and heat and stir to form a colorless and clear liquid as liquid A for later use; Then weigh scopolamine: 0.5g and add it to water, then add cyclodextrin: 3g, stir to make it dissolve into a colorless and clear solution, then add a part of polyethanol 400:14g and Span 60:3g, and it will be liquid B after dissolution At this time, heat the liquid A again, add liquid B while stirring to form a uniform emulsion, cool, stir to make the emulsion into a delicate cream, and then pack it into the warehouse.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com