Application of ring icariine in the preparation of medicine for preventing and treating organ transplantation rejection
A cyclic icariin and rejection technology is applied in the directions of drug combination, drug delivery, active ingredients of heterocyclic compounds, etc., to achieve good anti-rejection effect, inhibit rejection, and improve the function of transplanted organs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of cyclic icariin
[0018] (1) Preparation of icariin: method reported in literature [Yang Xiaoping, Chinese Pharmaceutical Journal, 1992, 28(1), 37; Li Wenkui et al., Northwest Pharmaceutical Journal, 1995, 10(3), 138], from Yin Icariin with a content of 90% is obtained by separating and purifying from the whole sheep herb.
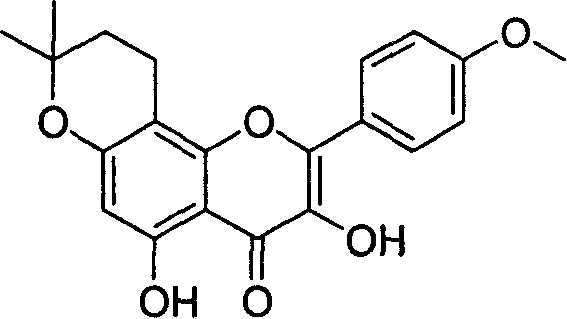
[0019] (2) Preparation of cyclic icariin (as shown in formula I): Dissolve 1 g of icariin in 50-500 mL of methanol, then add concentrated sulfuric acid with a volume of 5-30% of the solution, and heat to reflux 2-10 hours. After the reaction is completed, cool down and add 2-10 times of water, and a large amount of precipitation will be produced at this time. The precipitate was collected by filtration and washed with water until neutral. After the precipitate was dried, it was purified by silica gel column chromatography to obtain a light yellow solid product. The yield is 30-60%. The structure of the product has been ...
Embodiment 2
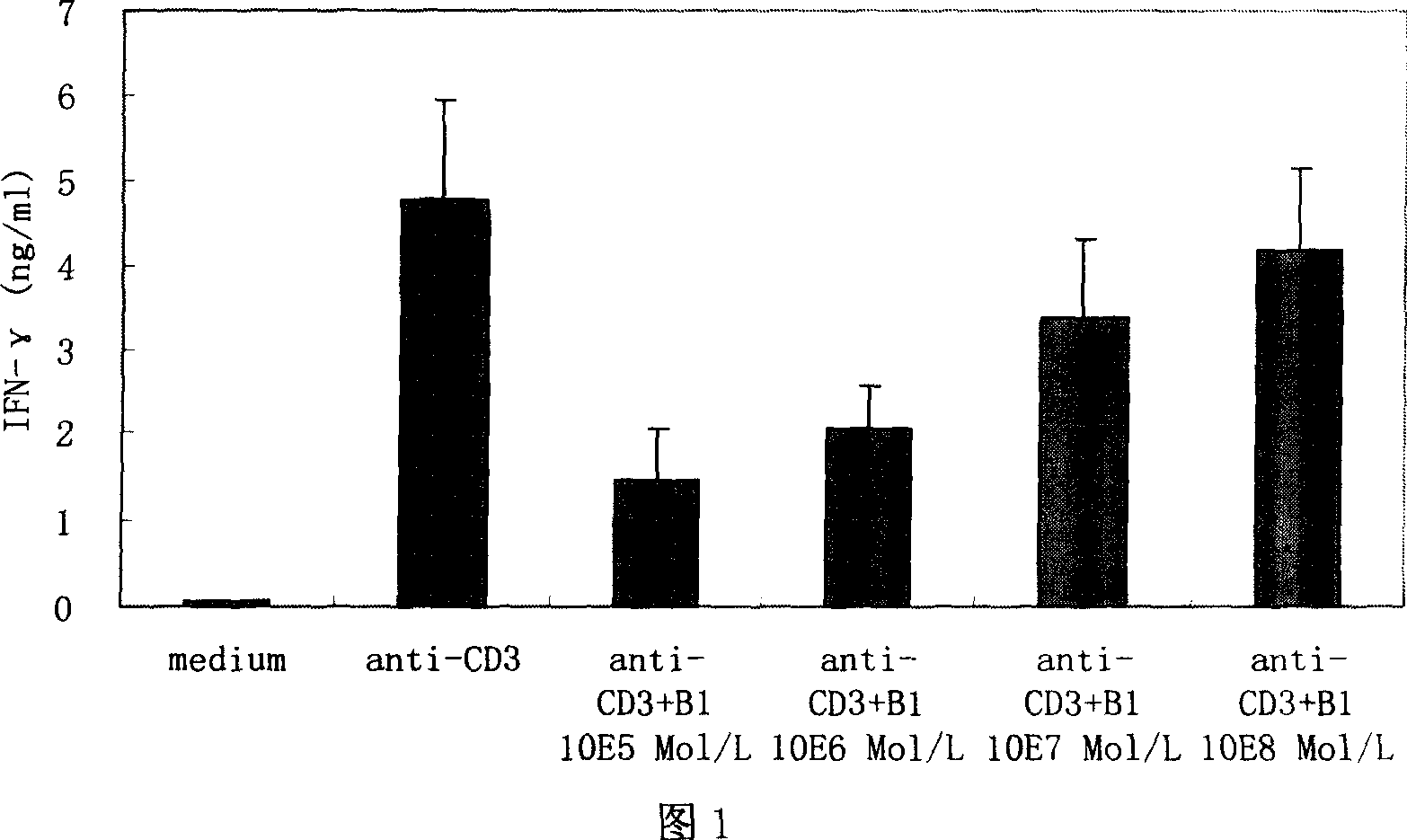
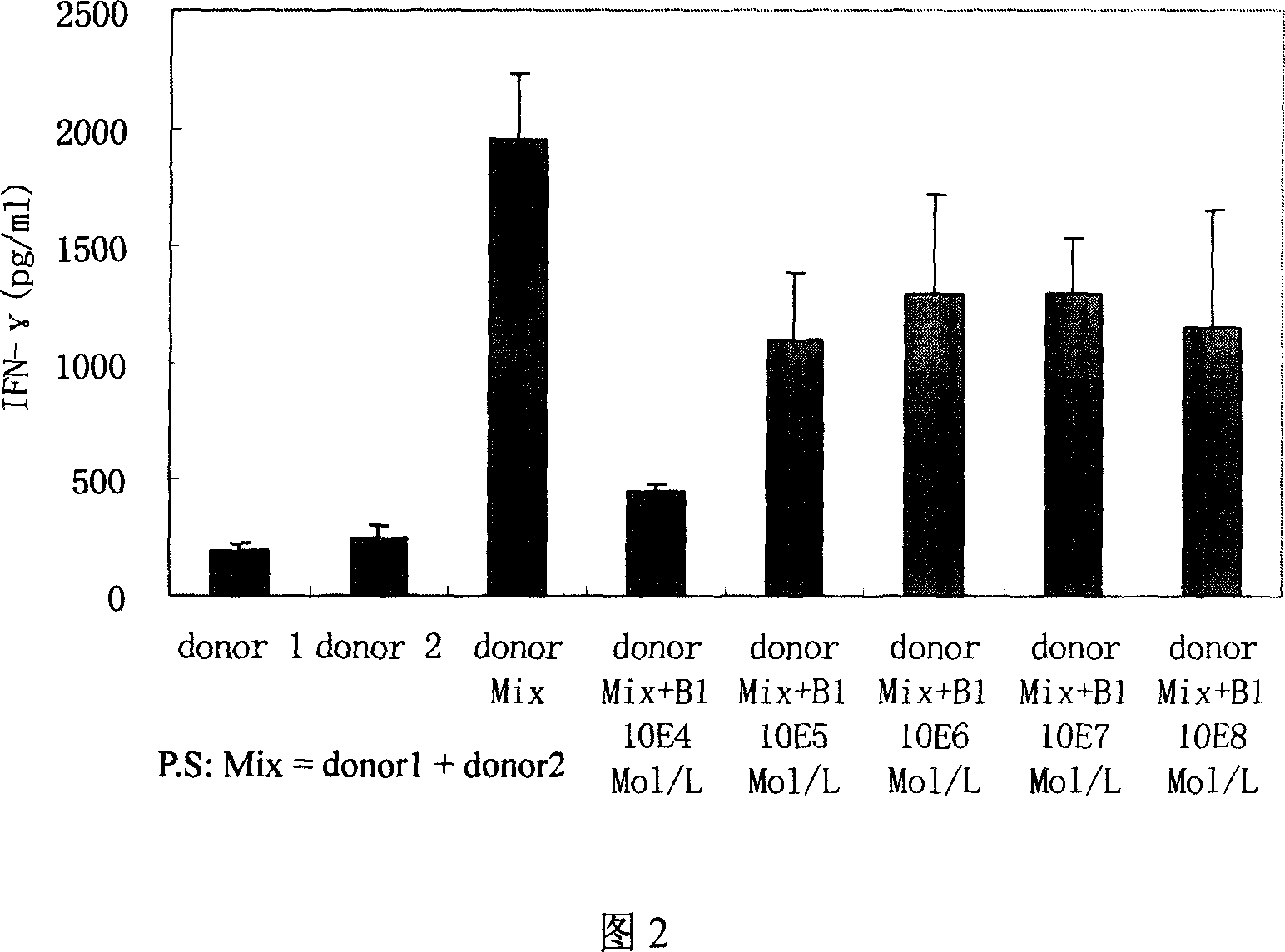
[0020] Example 2: In vitro immunosuppressive test of cyclic icarigenin
[0021] Experiment 1: Inhibitory effect of cyclic icario aglycone on normal human peripheral blood mononuclear cells (PBMCs) γ-interferon (interferon-gamma, IFN-γ)
[0022] (1) Isolation of normal human peripheral blood mononuclear cells (PBMCs)
[0023] The peripheral blood of normal people was taken for anticoagulation with heparin, diluted with an equal amount of Hank's buffer, mixed evenly, and layered on top of dextran-Ficoll-Hypaque. Centrifuge at 24°C for 25 minutes (2500 rpm). After centrifugation, draw the PBMCs layer, wash it twice with buffer, and then add RPMI1640 complete medium to suspend the cells.
[0024] (2) Cell culture
[0025] Adjust PBMCs to 2 × 10 6 Cells / ml, cultured in 96-well plate, 200uL per well, 3 replicate wells for each group. PBMCs added anti-CD3 monoclonal antibody (0.2 μg / mL) and anti-CD3 monoclonal antibody + different concentrations of cyclopicaridin, 5% CO 2 , 37 ...
Embodiment 3
[0030] Example 3: Cyclic icariin prevents and treats rejection in a rat heart transplant model
[0031] Test drug: cyclic icariin. Control drug: tacrolimus (FK506).
[0032] Test animals: Donor Brown Norway (BN, RT1 n ) rat; recipient Lewis (LEW, RT1 1 ) rats.
[0033] Experimental method: Allogeneic heart transplantation was performed among rats of different strains, and the allogeneic heart transplantation model was constructed. On the first day after operation, cyclic icariin and FK506 were administered orally to recipient rats respectively. The dose of cyclic icariin is 6-24 mg / kg per day, and the dose of FK506 is 0.5-1.5 mg / kg per day. The survival time of recipient rats after transplantation was observed.
[0034] test results:
[0035] Compared with the control group, the recipients of the rat heart transplantation model in the 6mg / kg day, 12mg / kg day, 24mg / kg day cyclic icariin and 1.0mg / kg day, 1.5mg / kg day FK506 groups The survival time of rats was significan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com