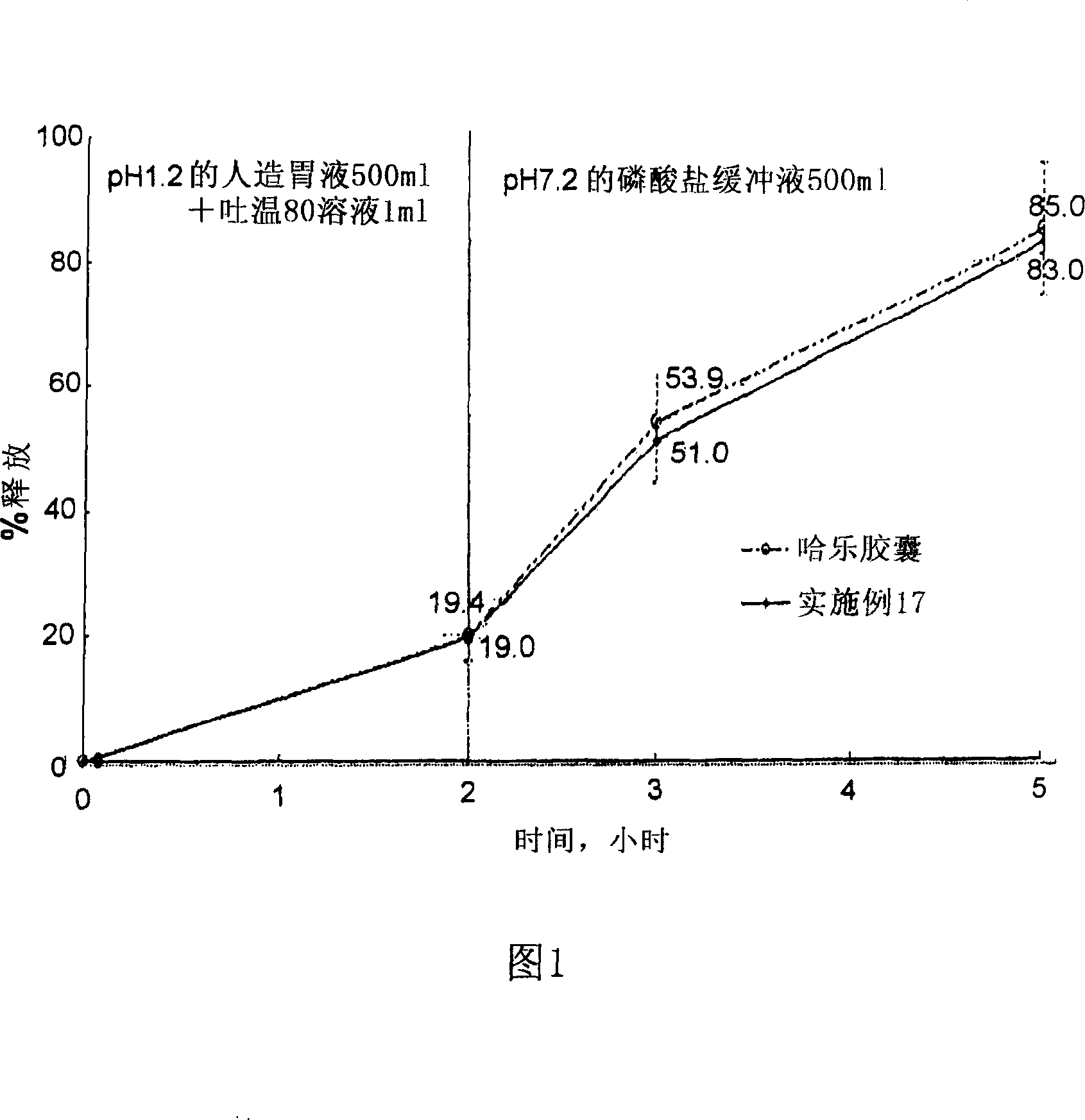
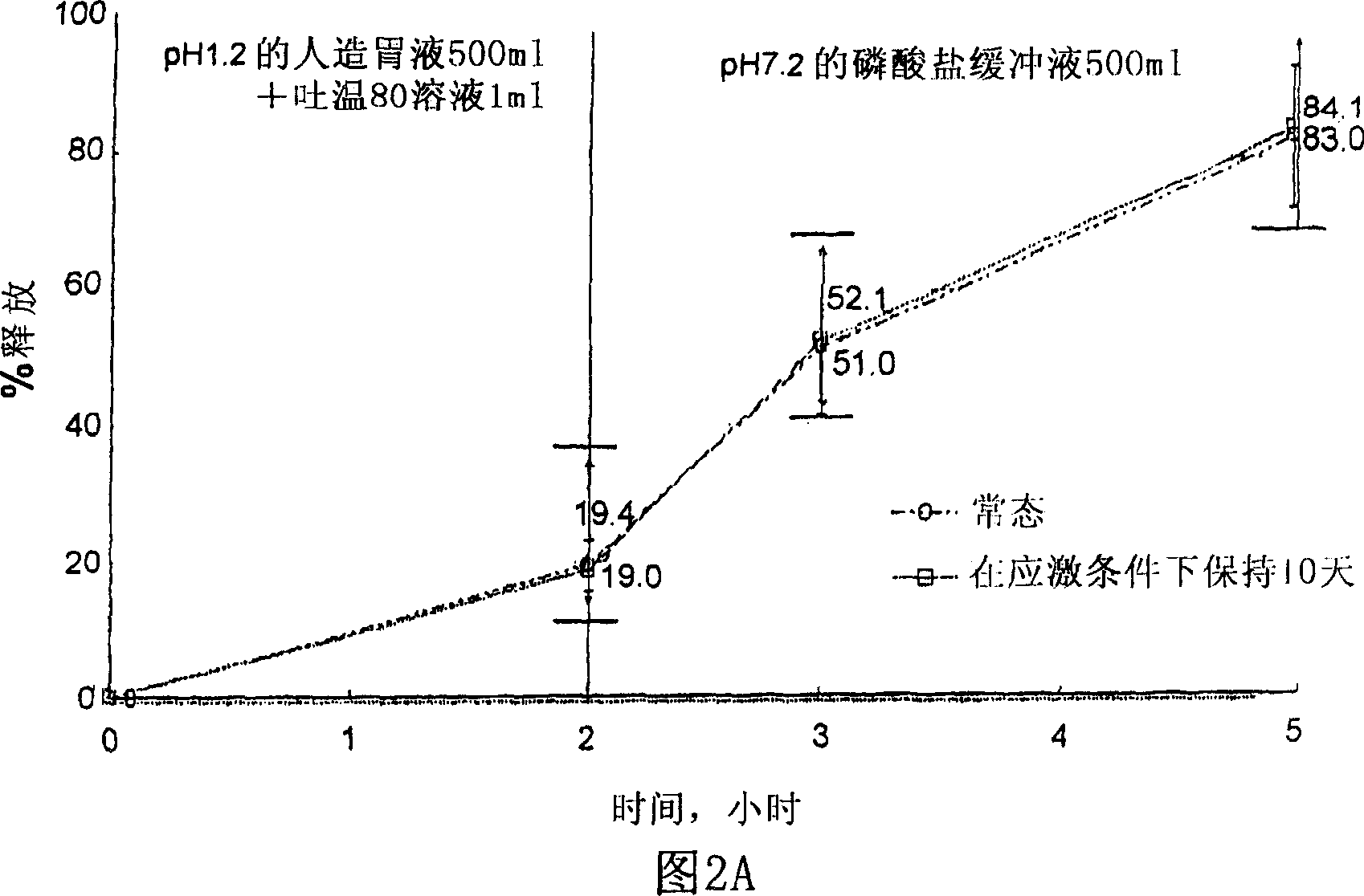
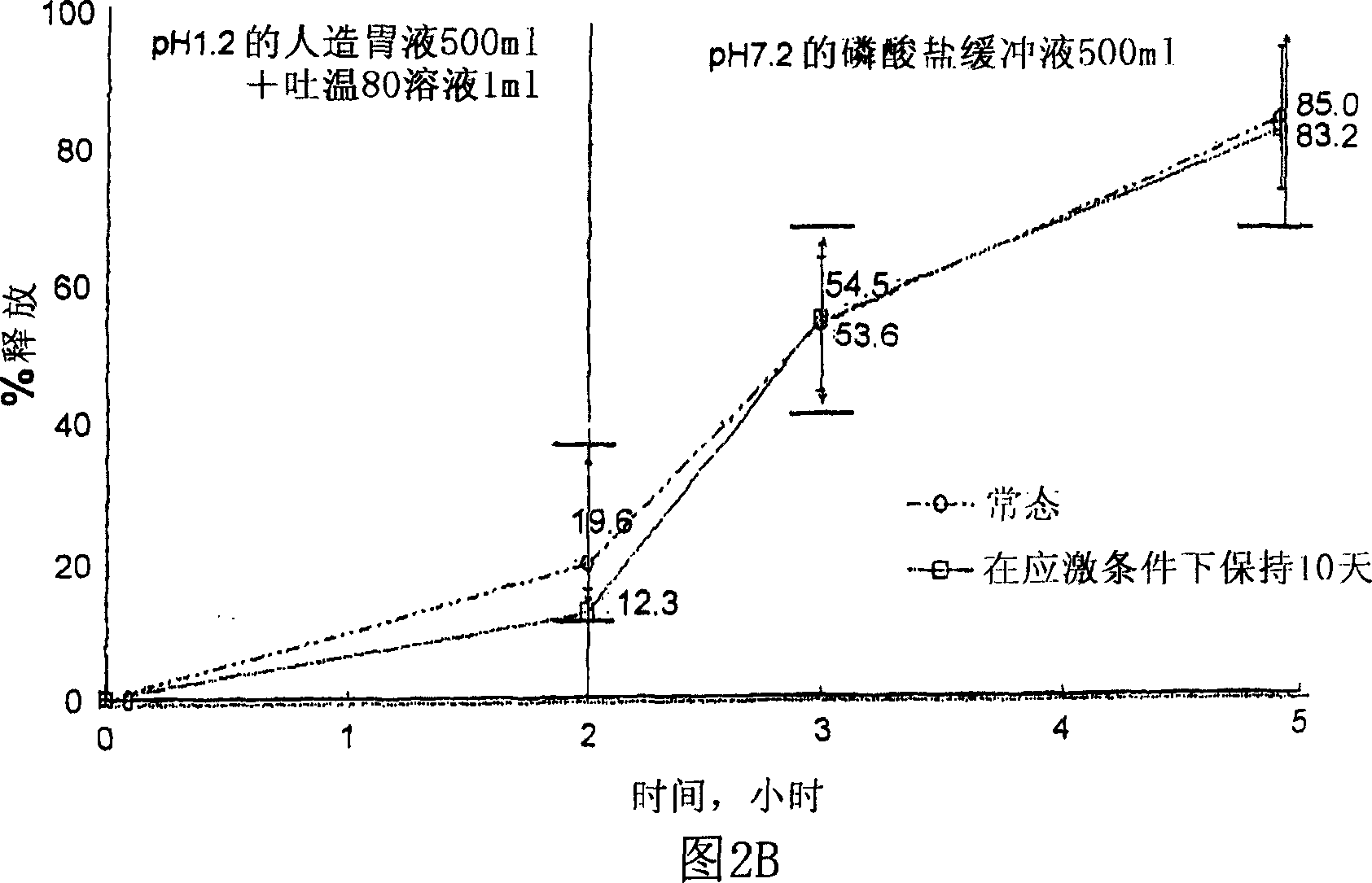
Composition for oral administration of tamsulosin hydrochloride and controlled release granule formulation comprising same
A technology of Tansylate hydrochloride and composition, which is applied in the direction of drug combination, medical preparation of non-active ingredients, drug delivery, etc., and can solve problems such as stability and release characteristics fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 0.2 parts by weight of tamsulosin hydrochloride, 21.0 parts by weight of Kollicoat SR30D (BASF, polyvinyl acetate), 5.5 parts by weight of METOLOSE 90SH (water-soluble HPMC; viscosity: 100,000 cps) and 123.5 parts by weight of microcrystalline fibers The vegetable (granulation agent) is placed in a high-speed mixer, and an appropriate amount of water is added to it. The mixture is mixed for 10-15 minutes and processed in a wet mill to obtain the ground material, which is passed through a compression mold equipped with a 0.8mm mesh, and granulated by a spheroidizer to obtain the required granules.
Embodiment 2
[0042] Except for using 133.5 parts by weight of dibasic calcium phosphate as a granulating agent, the steps of Example 1 were repeated to obtain the desired granules.
Embodiment 3
[0044] Except for using 95.5 parts by weight of dibasic calcium phosphate dihydrate as a granulating agent, the steps of Example 1 were repeated to obtain the desired granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com