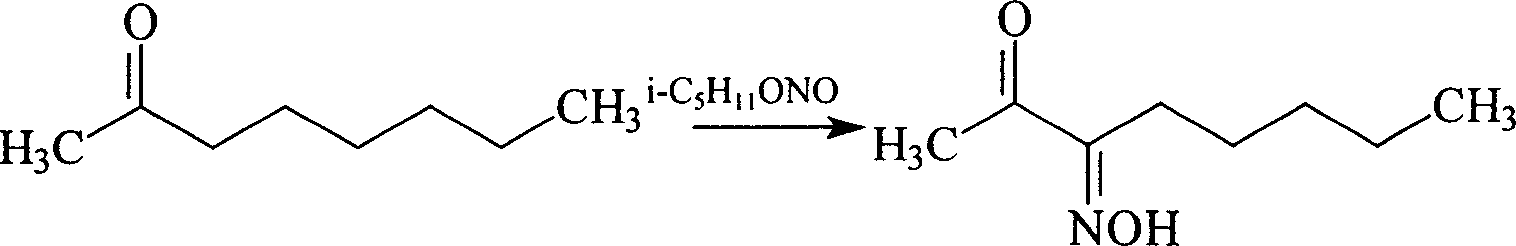Method for synthesis of 2,3-octadione monoxime
A technology of octanedione monoxime and a synthetic method, which is applied in two fields, can solve the problems of low product yield, and achieve the effects of high reaction yield, easy operation, and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0007] The implementation of the present invention will be further described below.
[0008] Add 166 grams (1.3mol) of 2-octanone and 12 milliliters of dilute hydrochloric acid into a 500ml round-bottomed three-neck flask equipped with a stirrer, dropping funnel, and thermometer, and start to drop 200 grams (1.7mol) of nitrous acid under cooling. For isoamyl ester, continue to stir for 2 hours after dropping, and keep the temperature between 40-50°C throughout. The reaction yield was 78.8%. And 2,3-octanedione monoxime is an important intermediate for the synthesis of 2,3-octanedione.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com