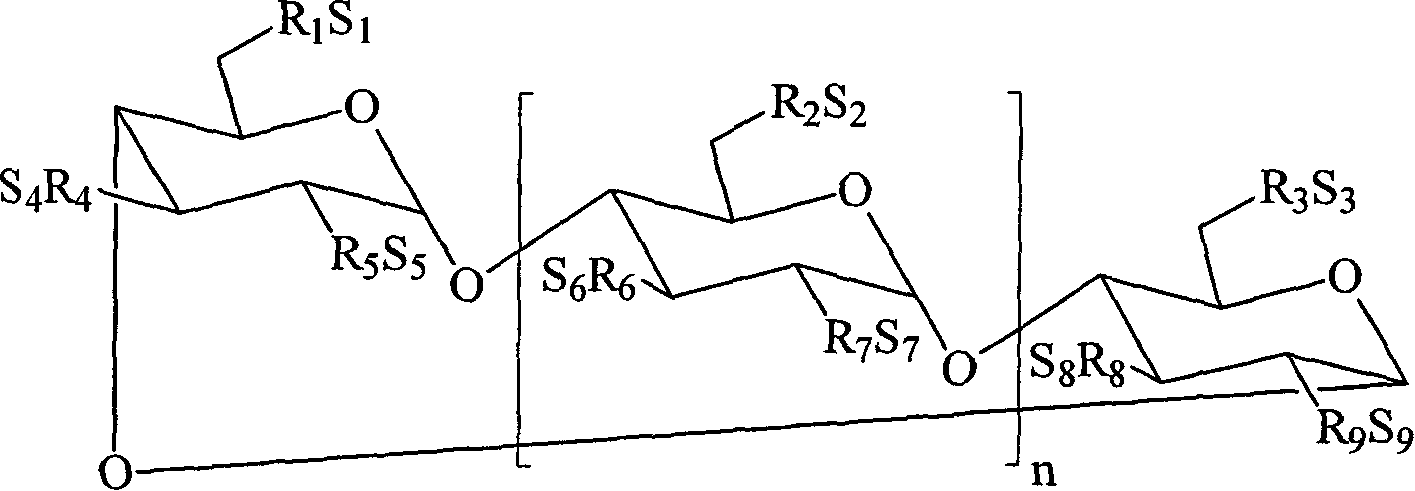
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
A technology of corticosteroids, preparations, applied in the field of treatment of lung diseases and disorders
Active Publication Date: 2007-06-06
CYDEX PHARMACEUTICALS INC
View PDF43 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
And the prior art does not suggest that one cyclodextrin-corticosteroid inhalable formulation would be advantageous over another cyclodextrin-corticosteroid inhalable formulation
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
An inhalable formulation containing SEA-gamma-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-gamma-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Description
Inhalation formulations containing sulfoalkyl ether cyclodextrins and corticosteroids Inventors: JamesD.Pipkin, RupertO.Zimmerer, DianeO.Thompson, GeroldL.Mosher technical field The present invention relates to a method and formulation for administering sulfoalkyl ether cyclodextrins and corticosteroids such as budesonide by inhalation. The present invention also relates to methods of treating pulmonary diseases and conditions. Background technique Drug delivery by inhalation results in drug deposition in various parts of the respiratory tract, such as the throat, trachea, bronchi, and alveoli. In general, the smaller the particle size, the longer the particles remain suspended in the air, so the drug can be delivered deeper into the respiratory tract. Corticosteroids are delivered by inhalation using a nebulizer, metered dose inhaler, or dry powder inhaler. The main advantages of nebulizers are that they do not require patient cooperation and are simpler to deliver hi...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/12A61K45/06C08B37/16A61K9/00A61K9/08A61K9/14A61K31/16A61K31/57A61K31/715A61K47/40A61K47/48
CPCA61K9/0078A61K31/16A61K47/48969A61K31/57A61K47/40A61K31/715B82Y5/00A61K9/08A61K9/0075A61K31/573A61K47/6951A61K31/137A61K31/167A61K31/46A61K31/56A61K31/58A61P11/00A61P11/06A61P29/00A61P43/00A61K2300/00A61K9/00
Inventor 詹姆斯·D.·皮普金鲁珀特·O.·齐默勒戴安娜·O.·汤普森格罗尔德·L.·莫舍
Owner CYDEX PHARMACEUTICALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com