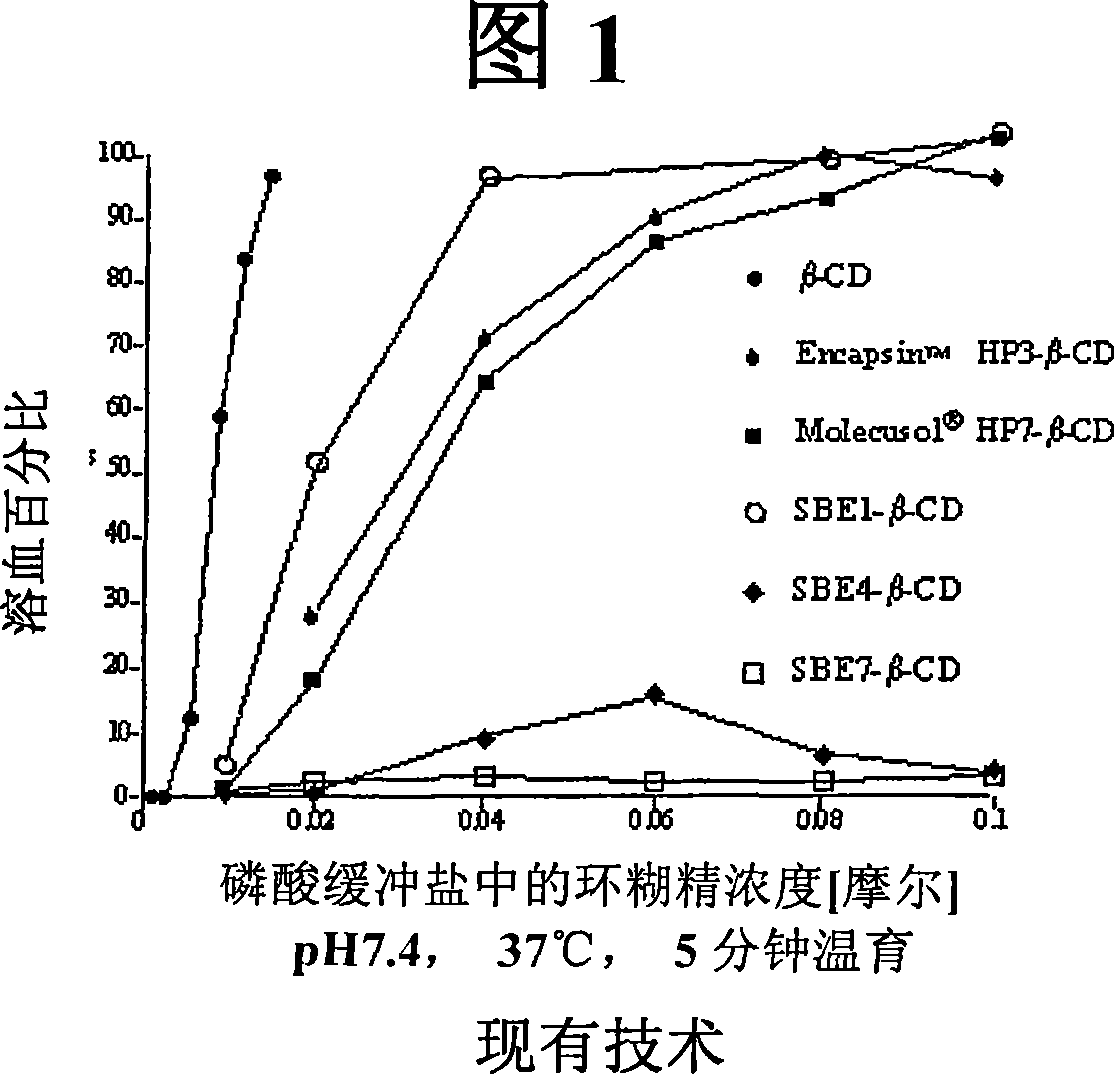
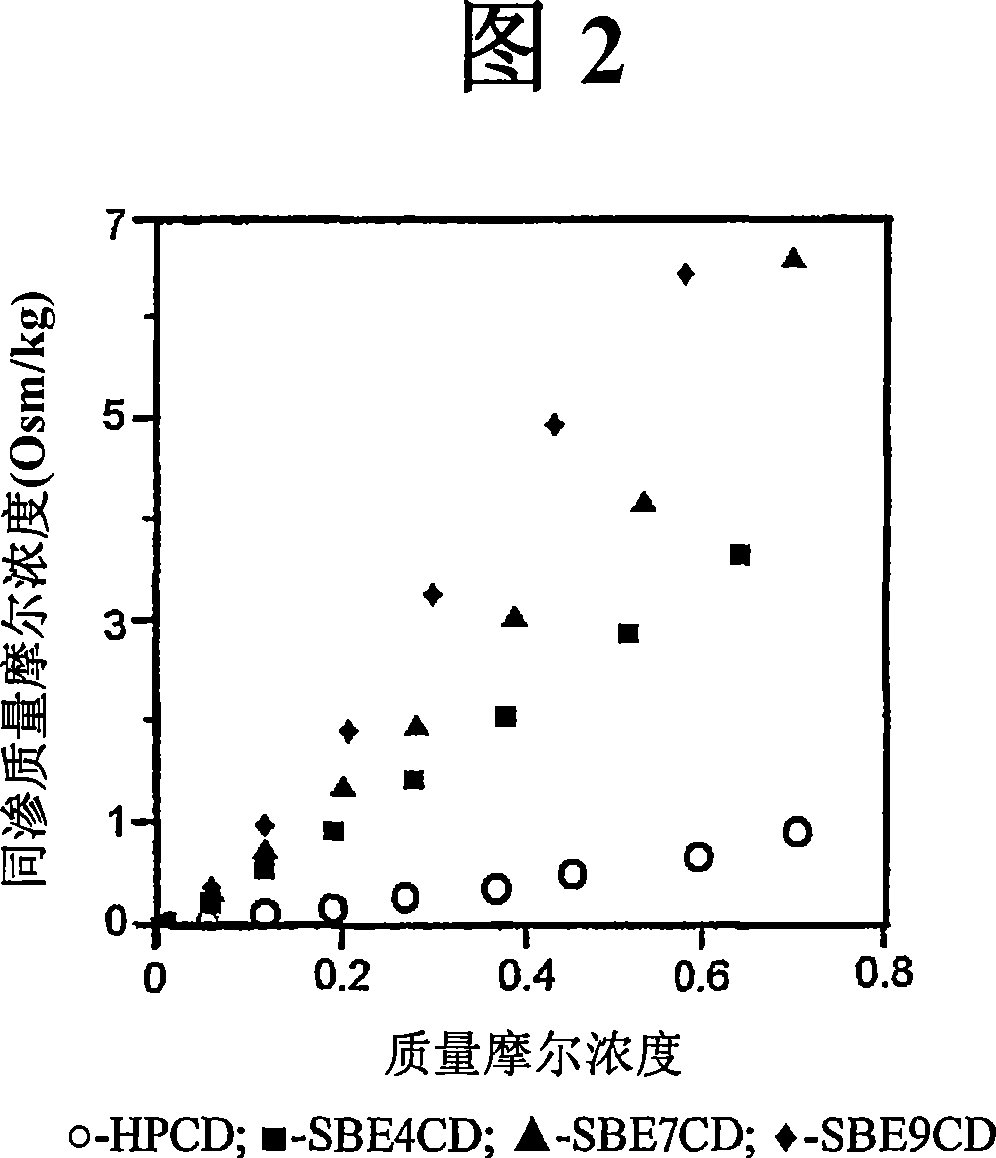
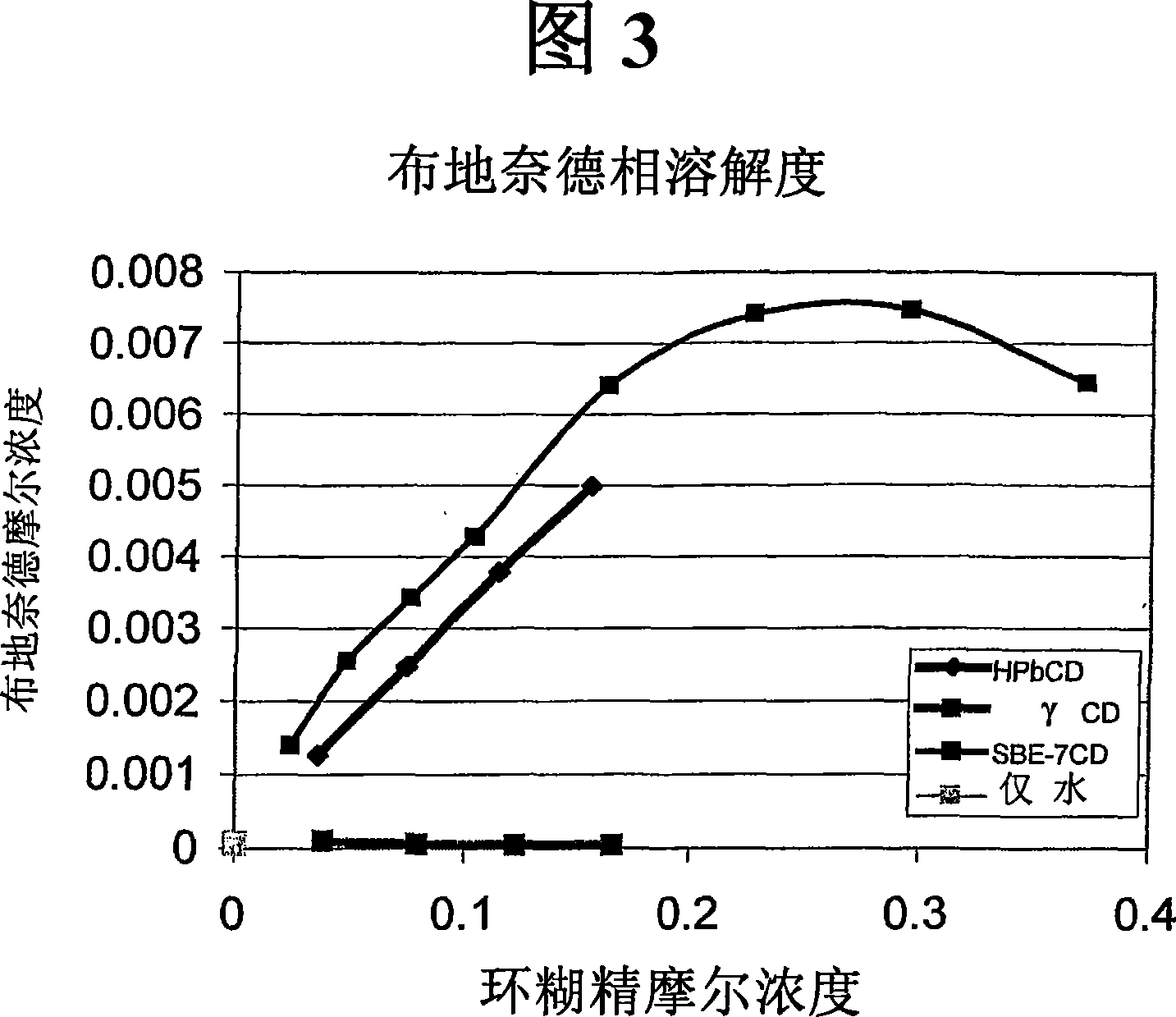
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
A corticosteroid, unit dose technology, applied in the direction of medical preparations containing active ingredients, anti-inflammatory agents, aerosol delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] Exemplary formulations of the invention are prepared according to the following general methods.
[0249] Method A
[0250] The cyclodextrin is dissolved in water (or buffer) to form a solution containing a known concentration of cyclodextrin. This solution is mixed with a solid, suspension, gel, liquid, paste, powder or other form of active substance, optionally with heating, to form an inhalable solution.
[0251] Method B
[0252] A known amount of substantially dry cyclodextrin is mixed with a known amount of substantially dry active. A liquid is added to this mixture with stirring to form a suspension, gel, solution, syrup or paste, optionally while heating and optionally in the presence of one or more other excipients to form a Solution for inhalation.
[0253] Method C
[0254] A known amount of substantially dry cyclodextrin is added to a suspension containing a known amount of active s...
Embodiment 2
[0257] The MMD of nebulized solutions containing SBE7-[beta]-CD and budesonide was determined as follows.
[0258] Three different cyclodextrin placebo solutions were prepared at different concentrations. 2 mL of the solution was added to the cup of a Pari LC Plus nebulizer supplied with air from a Pari Proneb Ultra compressor. The particle size of the ejected droplets was determined using a Malvern Mastersizer S laser light scattering device.
Embodiment 3
[0260] The stability of the liquid formulations containing SAE-CD was determined by HPLC chromatographic analysis of aliquots drawn periodically from the stock solution.
[0261] By mixing various ratios of 0.01M citric acid with 0.02M Na 2 HPO 4 Mix to prepare citrate phosphate (McIlvaines) buffer at pH 4, 5, 6, 7 or 8. These stock solutions contained 5% by weight Captisol. Budesonide was dissolved at approximately 250 μg / mL in each buffer. Aliquots of the solution were stored at 40°C, 50°C and 60°C. Control samples were stored at 5°C but are not reported here. HPLC analysis of samples was performed initially and after 1, 2 and 3 months of storage.
[0262] equipment
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com