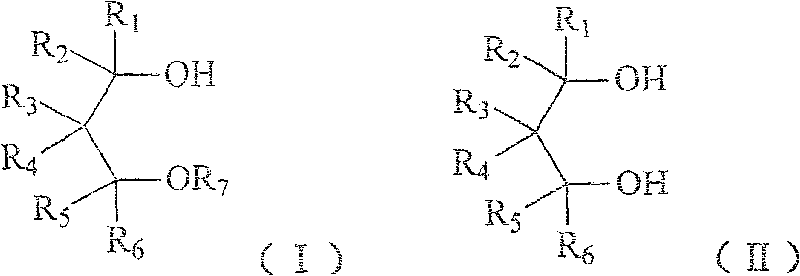
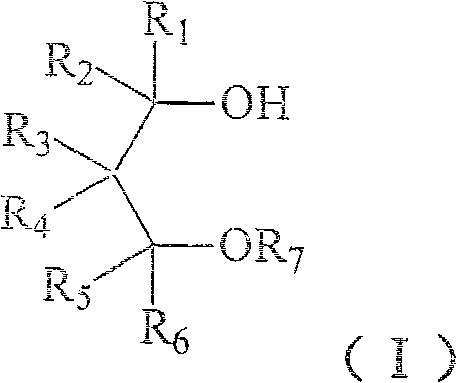
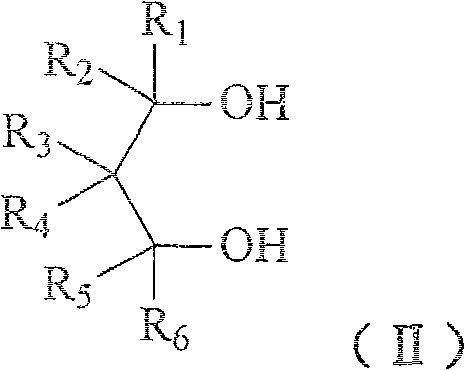
Method for preparing 1,3-glycol monoether compound
A technology of diol monoethers and compounds, which is applied in the direction of ester reaction to prepare ethers, ether preparations, organic chemistry, etc., can solve the problems of cumbersome operation, hidden safety hazards, and expensive halogenated alkanes, and achieves mild reaction conditions, easy separation and purification, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 2, the preparation of 2-dimethyl-3-methoxy-1-propanol
[0051] Under stirring, 10.4 g of 2,2-dimethyl-1,3-propanediol, 7.2 g of sodium hydroxide, 0.5 g of benzyltrimethylammonium chloride and 16.8 g of water were uniformly mixed, and the temperature was raised to 90°C. 18.9 g of dimethyl sulfate was added dropwise, and reacted at 90° C. for 3 hours after the drop was completed. The product was cooled, and the oil layer was separated, and the water layer was taken with dichloromethane benzene and combined with the oil layer. The dichloromethane was distilled off, distilled under reduced pressure, and the 58-75°C / 15mmHg fraction was collected to obtain 7.8 g of colorless and transparent 2,2-dimethyl-3-methoxy-1-propanol with a yield of 66%.
[0052] 1H NMR (CDCl3 / TMS) δ (ppm): 0.92 (s, 6H, CH3), 2.65 (t, 1H, OH), 3.24 (s, 2H, CH2), 3.34 (s, 3H, CH3), 3.44 ( s, 2H, CH2).
Embodiment 2
[0053] Example 2 2, the preparation of 2-dimethyl-3-ethoxyl-1-propanol
[0054] Under stirring, 5.2g of 2,2-dimethyl-1,3-propanediol, 3g of sodium hydroxide, 0.3g of benzyltriethylammonium chloride and 7.0g of water were uniformly mixed, and the temperature was raised to 60°C. 11.6 g of diethyl sulfate was added dropwise, and reacted at 60° C. for 5 hours after the drop was completed. The product was cooled, and the oil layer was separated, and the water layer was extracted with ether and combined with the oil layer. Distill off ether, distill under reduced pressure, collect fractions at 70-80°C / 15mmHg to obtain 4.2 g of colorless and transparent 2,2-dimethyl-3-ethoxy-1-propanol with a yield of 64%.
[0055] 1H NMR (CDCl3 / TMS) δ (ppm): 0.92 (s, 6H, CH3), 1.19 (t, 3H, CH3), 2.98 (t, 1H, OH), 3.29 (s, 2H, CH2), 3.44- 3.51 (m, 4H, CH2).
Embodiment 3
[0056] Example 3 Preparation of 2,2-diisobutyl-3-methoxy-1-propanol
[0057] Under stirring, 1.9g 2,2-diisobutyl-1,3-propanediol, 0.6g sodium hydroxide, 0.1g benzyltriethylammonium chloride, 1.4g water and 10ml benzene were mixed uniformly, and the temperature was raised to 60°C. 1.9 g of dimethyl sulfate was added dropwise and reacted at 60° C. for 5 hours after the drop was completed. Cool the product, separate the oil layer, evaporate the benzo and distill under reduced pressure, collect fractions at 120-125°C / 140Pa to obtain colorless and transparent 2,2-diisobutyl-3-methoxy-1-propanol 1.4 g, yield 69%.
[0058] 1H NMR (CDCl3 / TMS) δ(ppm): 0.91~0.95(m, 12H, CH3), 1.28(d, 4H, CH2), 1.66-1.72(m, 2H, CH), 2.79(t, 1H, OH ), 3.32 (S, 3H, CH3), 3.33 (S, 2H, CH2), 3.53 (d, 2H, CH2).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap