Chromatography column qualification in manufacturing methods for producing anti-IL12/IL23 antibody compositions
a technology of chromatography column and manufacturing method, which is applied in the field of chromatography column, can solve the problems of increasing column diameter, equipment and buffer consumption, and possible changes in the integrity of packed beds, and achieves the effect of reducing the quality of the chromatography column packing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of the Gamma Distribution Transition Analysis for Column Qualification of Protein A Chromatography Columns Used in to REMICADE® (Infliximab) Manufacturing
Overview:
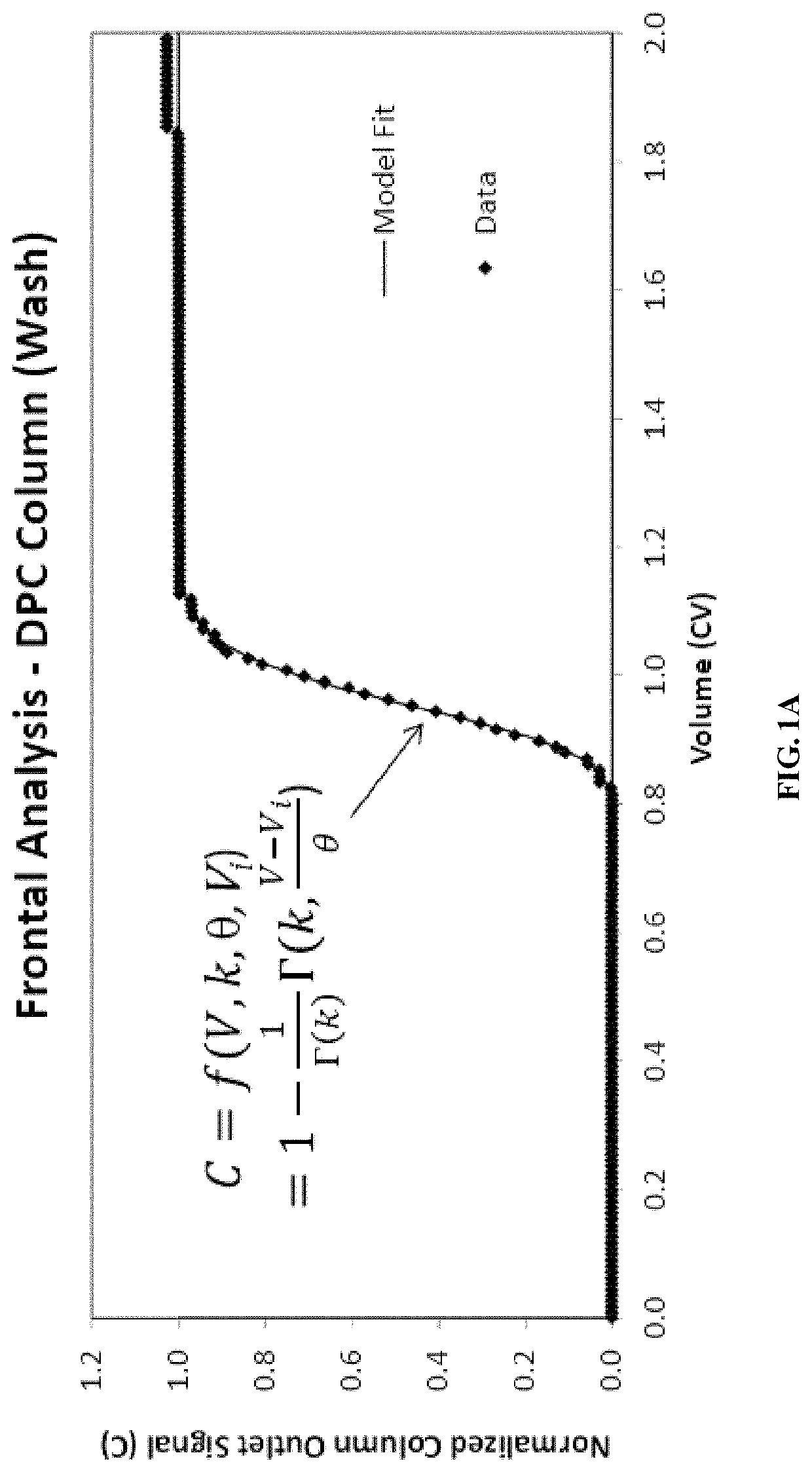
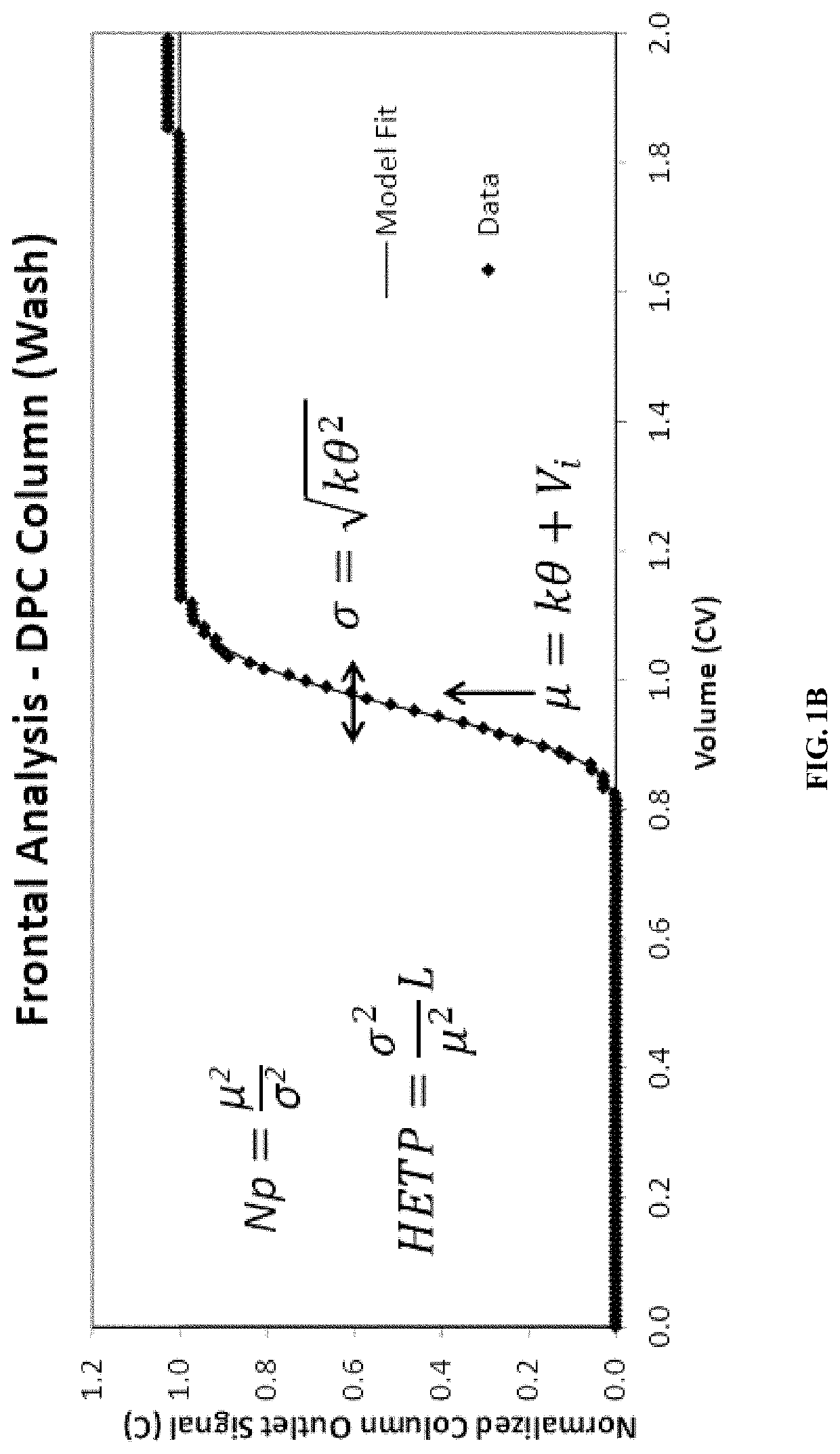
[0161]The manufacturing process of the therapeutic antibody, REMICADE® (infliximab), involves several stages, four of which involve chromatography purification. The gamma distribution transition analysis (GDTA) for column qualification was applied to two or three transitions during each of these column steps. This Example describes the application of the GDTA method to the Protein A column purification step employed during REMICADE® (infliximab) manufacturing. The purification process includes two transition fronts, i.e., equilibration and intermediate wash, that are appropriate for GDTA as described herein.
[0162]The GDTA was executed on 129 fronts from the consecutive purification of 69 batches of REMICADE® (infliximab), comprising 60 equilibration and 69 wash fronts. The gamma front distribution analysis was performed...
example 2
on of the Gamma Distribution Transition Analysis for Detection of Sub-Optimal Performance of Protein A Chromatography Columns Used in to REMICADE® (Infliximab) Manufacturing
[0189]The manufacturing process of the therapeutic antibody, REMICADE® (infliximab), involves several stages, four of which involve chromatography purification. The gamma distribution transition analysis (GDTA) for column qualification was applied to two or three transitions during each of these column steps. This Example describes the application of the GDTA method to the Protein A column purification step employed REMICADE® (infliximab) manufacturing. The purification process includes two transition fronts, i.e., equilibration and intermediate wash, that are appropriate for GDTA as described herein.
[0190]The GDTA was executed on 45 Equilibration fronts from the consecutive purification of 45 batches of REMICADE® (infliximab), comprising 23 batches processed on column pack 883333M001 and 22 batches processed on ...
example 3
on of the Gamma Distribution Transition Analysis for Column Qualification of SP-Sepharose High Performance Chromatography Columns Used in to REMICADE® (Infliximab) Manufacturing
Overview:
[0192]As discussed above, the manufacturing process of REMICADE® (infliximab) involves several stages, four of which involve chromatography purification. This Example describes the application of the GDTA method to the SP-Sepharose High Performance (SPHP) column purification step employed REMICADE® (infliximab) manufacturing. The SPHP column is a cation exchange chromatography column. The purification process includes three transition fronts, i.e., equilibration, WFI flush, and storage fronts, that are appropriate for GDTA as described herein.
[0193]The GDTA was executed on 69 fronts from the purification of 23 batches of REMICADE® (infliximab), comprising 23 equilibration, WFI flush, and storage fronts. The gamma front distribution analysis was performed concurrently with manufacturing and did not im...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com