Process for the production of HCMV glycoproteins, antibodies thereto and HCMV vaccines, and recombinant vectors therefor
a technology of hcmv glycoprotein and hcmv vaccine, which is applied in the field of process for the production of hcmv glycoprotein, antibodies thereto and hcmv vaccine, and recombinant vectors therefor, which can solve the problems of unsatisfactory side effects, unspecified total number of hcmv-specified glycoproteins, and uncertainty of the vaccine potential of individual glycoproteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Identification of Putative Glycoprotein Genes
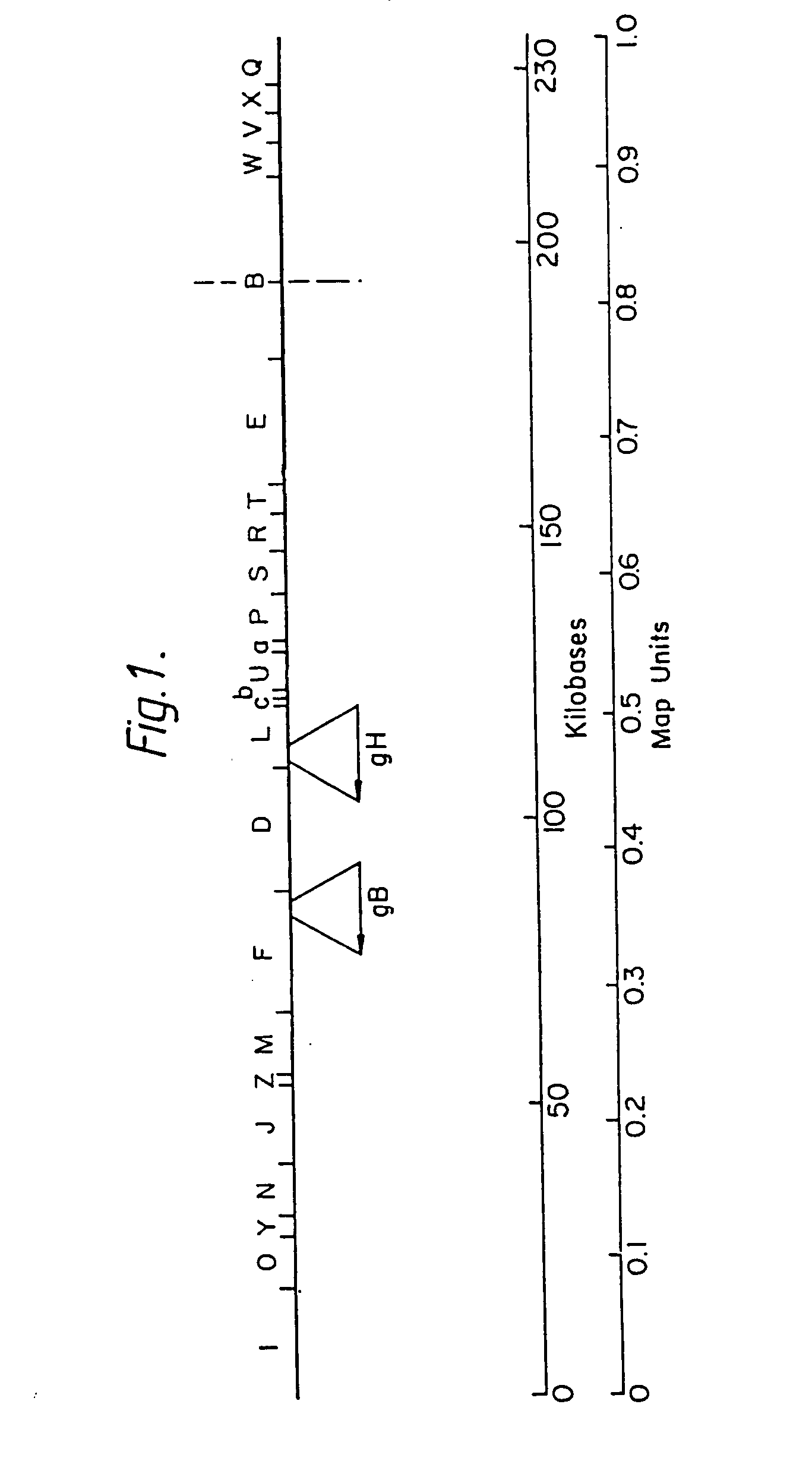
[0024] In order to look for possible glycoprotein genes within the HCMV genome individual cloned restriction fragments of the genome of HCMV strain AD169 were sequenced using the M13 / dideoxynucleotide chain termination method described in reference (11) using the strategy and methods described by Bankier and Barrell in reference (12). The resulting compiled sequences were then analysed for possible protein coding sequences and RNA polymerase II transcription signals. The predicted translation products of likely protein coding sequences were then examined for the presence of glycoprotein characteristics, namely an N-terminal hydrophobic signal peptide, a hydrophobic transmembrane sequence close to the C-terminus, and potential N-glycosylation sites in the external domain.
[0025] Using these criteria two putative glycoprotein genes were identified lying between bases 16255 and 18972 of the HindIII F fragment and between bases 228 and 2456 of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com