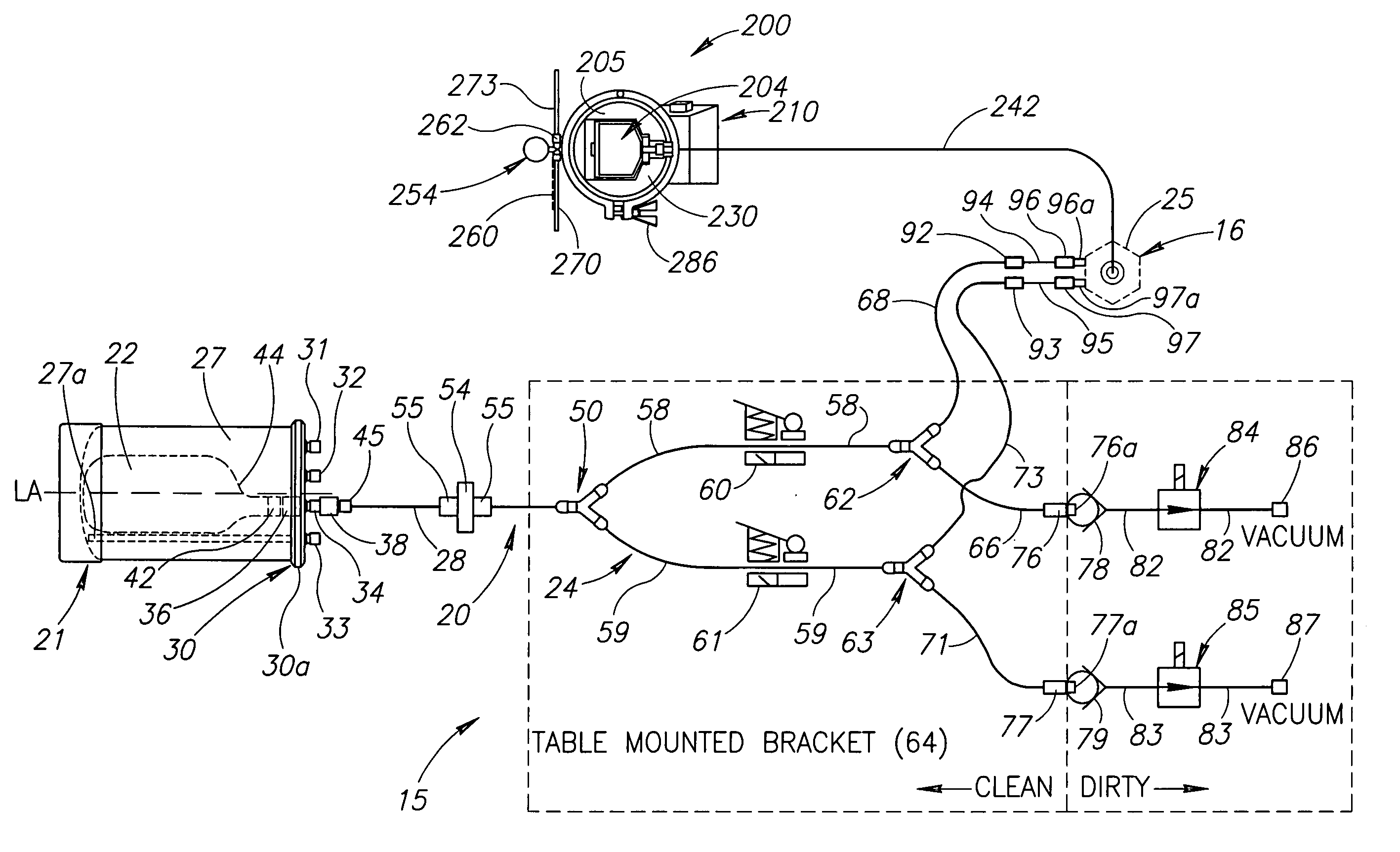
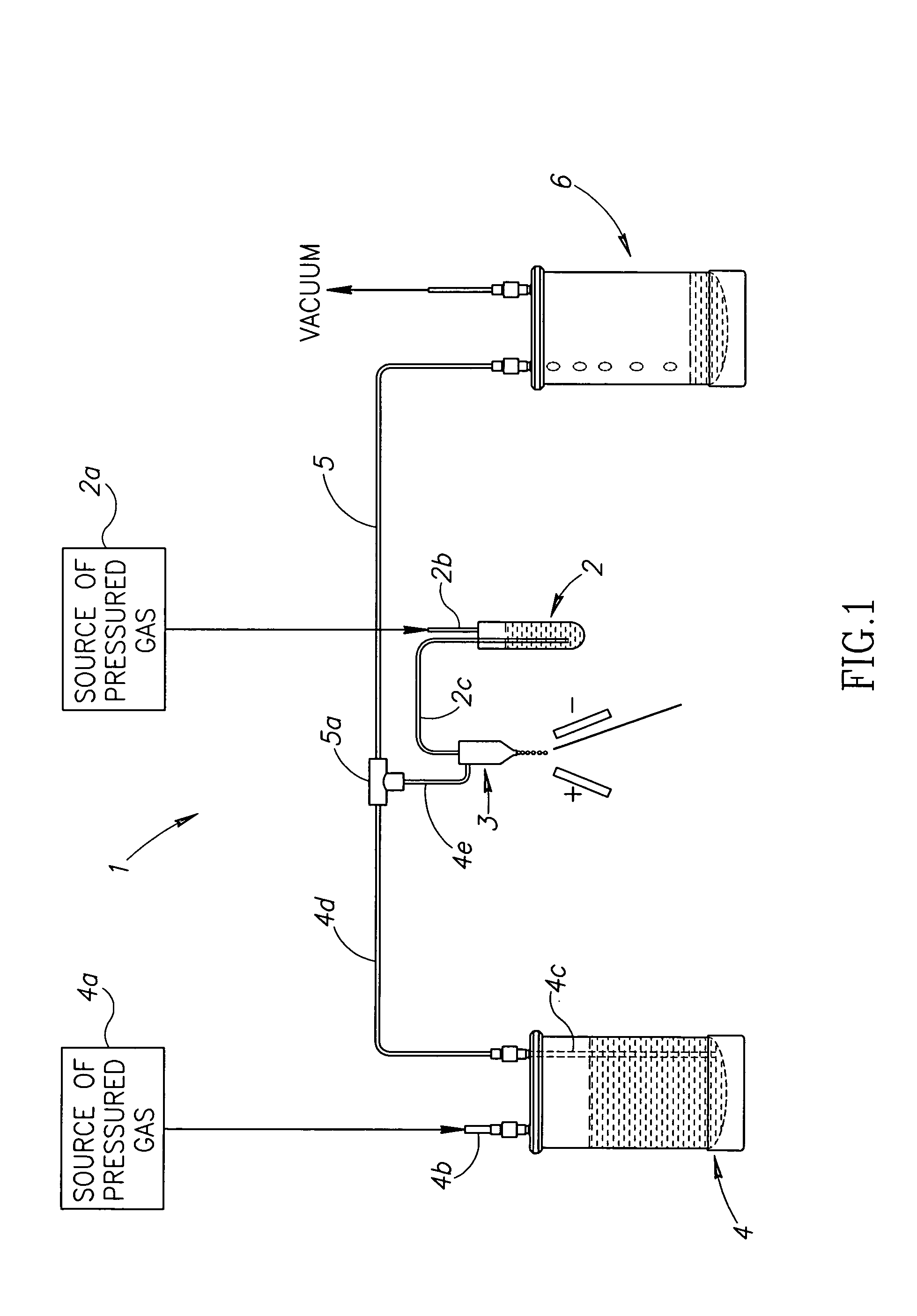
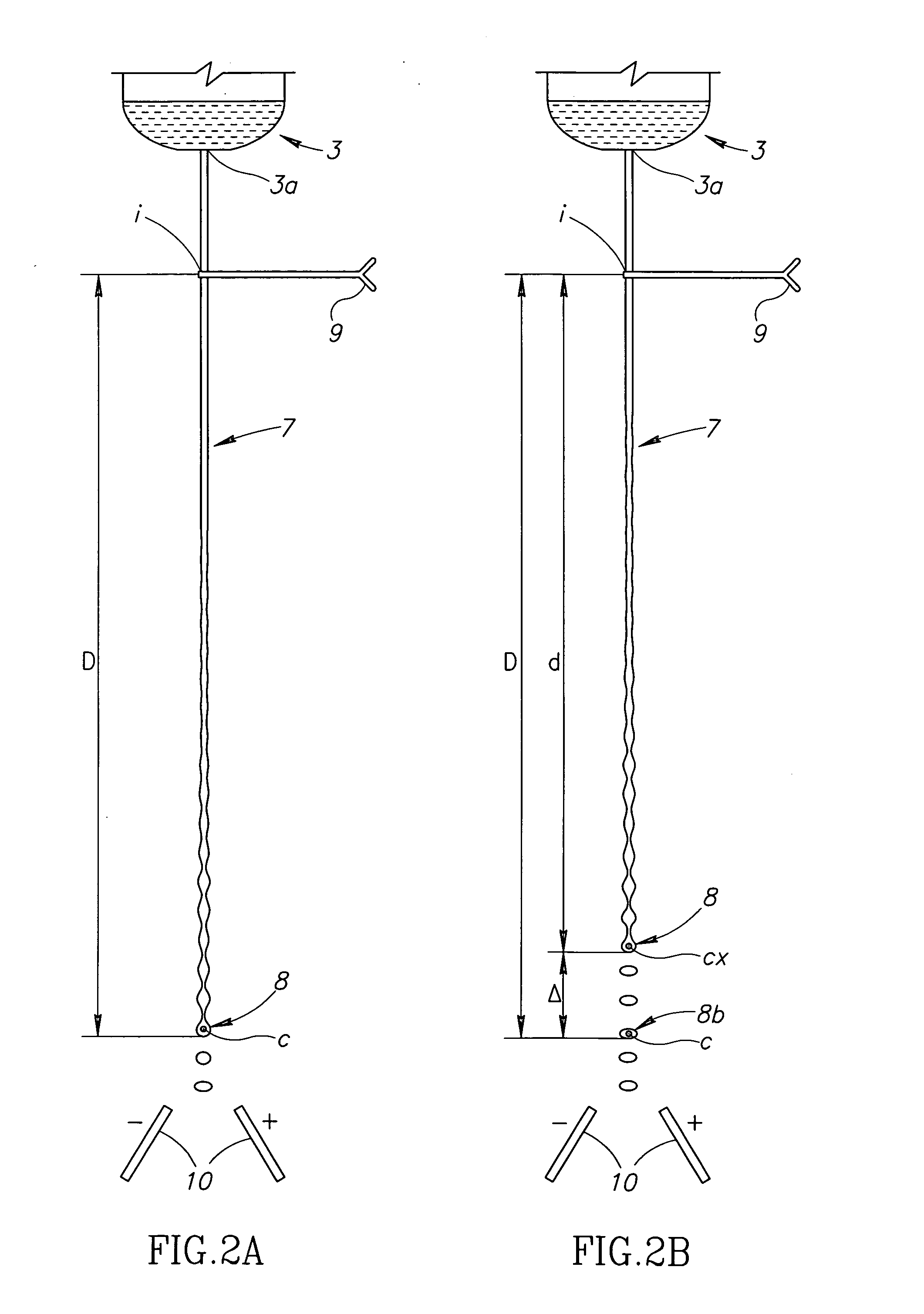
Fluid delivery system for a flow cytometer
a flow cytometer and flow cytometer technology, applied in the direction of instruments, packaging, transportation and packaging, etc., can solve the problems of adversely affecting the results of a study, contaminating the sample fluid, and altering the character of the cells being examined, etc., to achieve easy and secure coupling, easy fitting, and easy uncoupling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0086] A sheath tank, a stainless steel cylindrical tank with an approximately 8 inch diameter, a depth of at least 13⅜ inches, and able to withstand pressures of up to 100 psi when capped, was modified with an internal cylindrical tube (straw) that extended approximately 13 inches into the tank, to a point just above the floor. The tank was filled with approximately 8 liters of Dulbecco's Phosphate Buffered Saline (PBS). The cap had connections for pressure in, fluid out, and a pressure gauge. The pressure gauge included a differential pressure measuring (DPM) unit, that attached between the pressure in and fluid out connections. The differential pressure gauge measured the difference between gas pressure at the top of the tank and pressure of the fluid as it leaves the tank. The resultant readings are shown in FIG. 11A in a diagram of differential pressure (in pounds per square inch (psi) versus time (in minutes)).
[0087] The tank was sealed with the cap, so as to be air and water...
example 2
[0093] The tank of Example 1 was used, with the internal cylindrical tube removed, and a 5 liter bag of Dulbecco's PBS was placed into the interior of the tank. A Plexiglas board was also placed into the tank. The bag, at a line extending from the bag, was attached to the fluid out port, this fluid out port being the port that formerly accommodated the internal cylindrical tube (straw). This port also included a quick connect on the outside of the tank cover. The cap was sealed on the body of the tank, such that the tank was air and water-tight.
[0094] The tank was connected to the tubing set 24 as detailed above for Example 1. The tank was tilted to a horizontal position, where the bag rested on the Plexiglas board and the line that extended from the bag was also supported by this Plexiglas board. The tank was oriented so that the fluid out port, that accommodated the line that extended from the bag, was at a six-o'clock position. The tank was pressurized to approximately 60 psi us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressures | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com