Embolization device and a method of using the same
a technology of embolization device and embolization method, which is applied in the direction of dilators, prostheses, blood vessels, etc., can solve the problems of vascular spasm, paralysis or other adverse events, toxic to tissue, etc., and achieve the effect of reducing increasing the flexibility of the space-occupying devi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
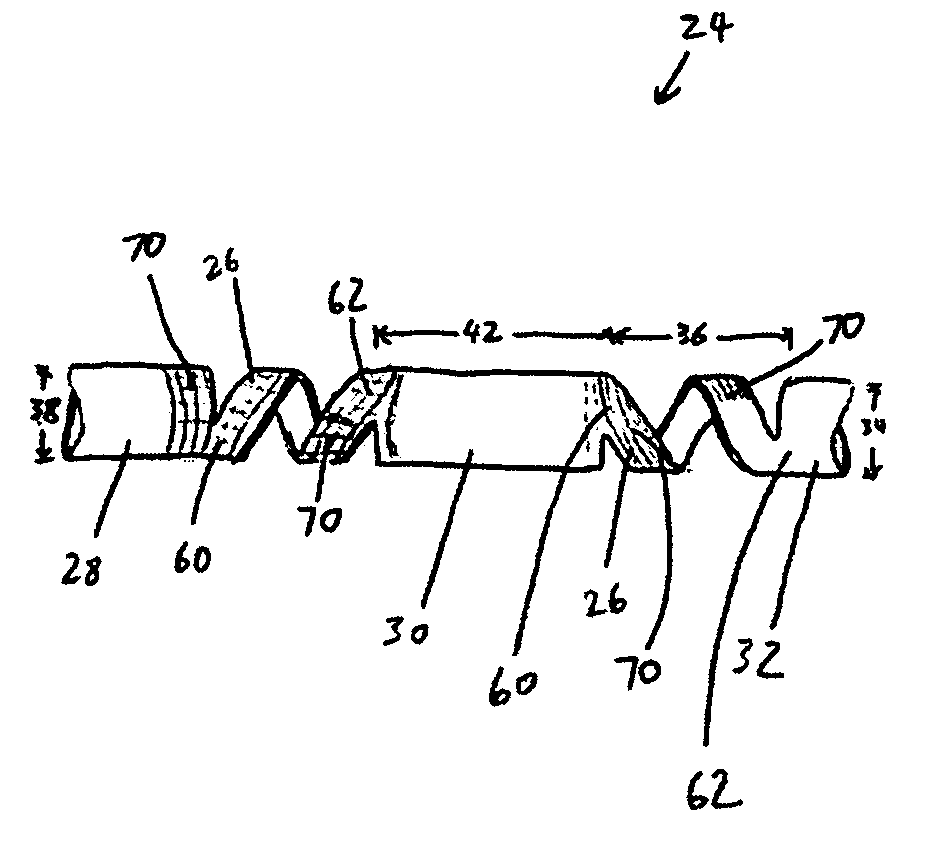
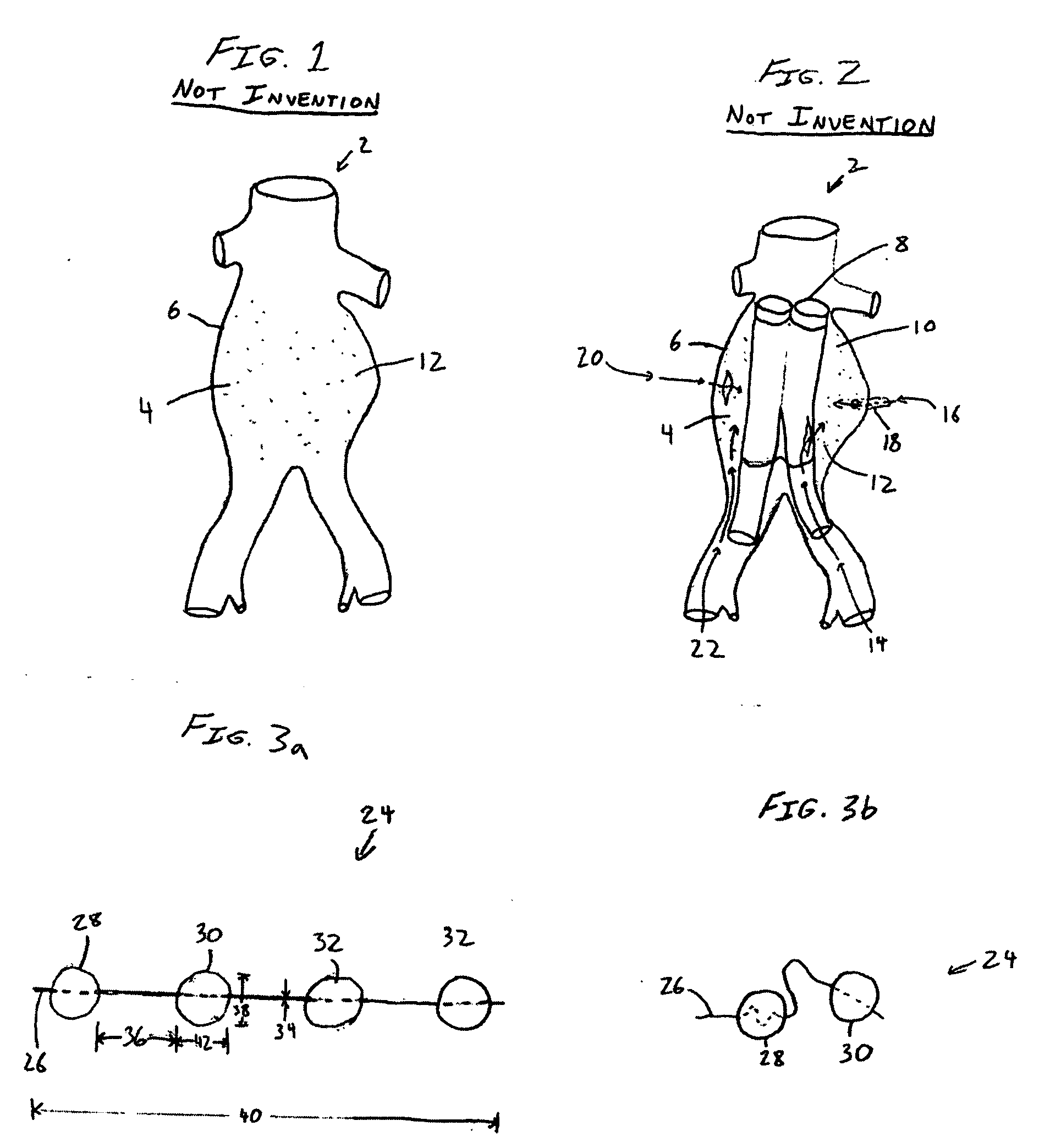
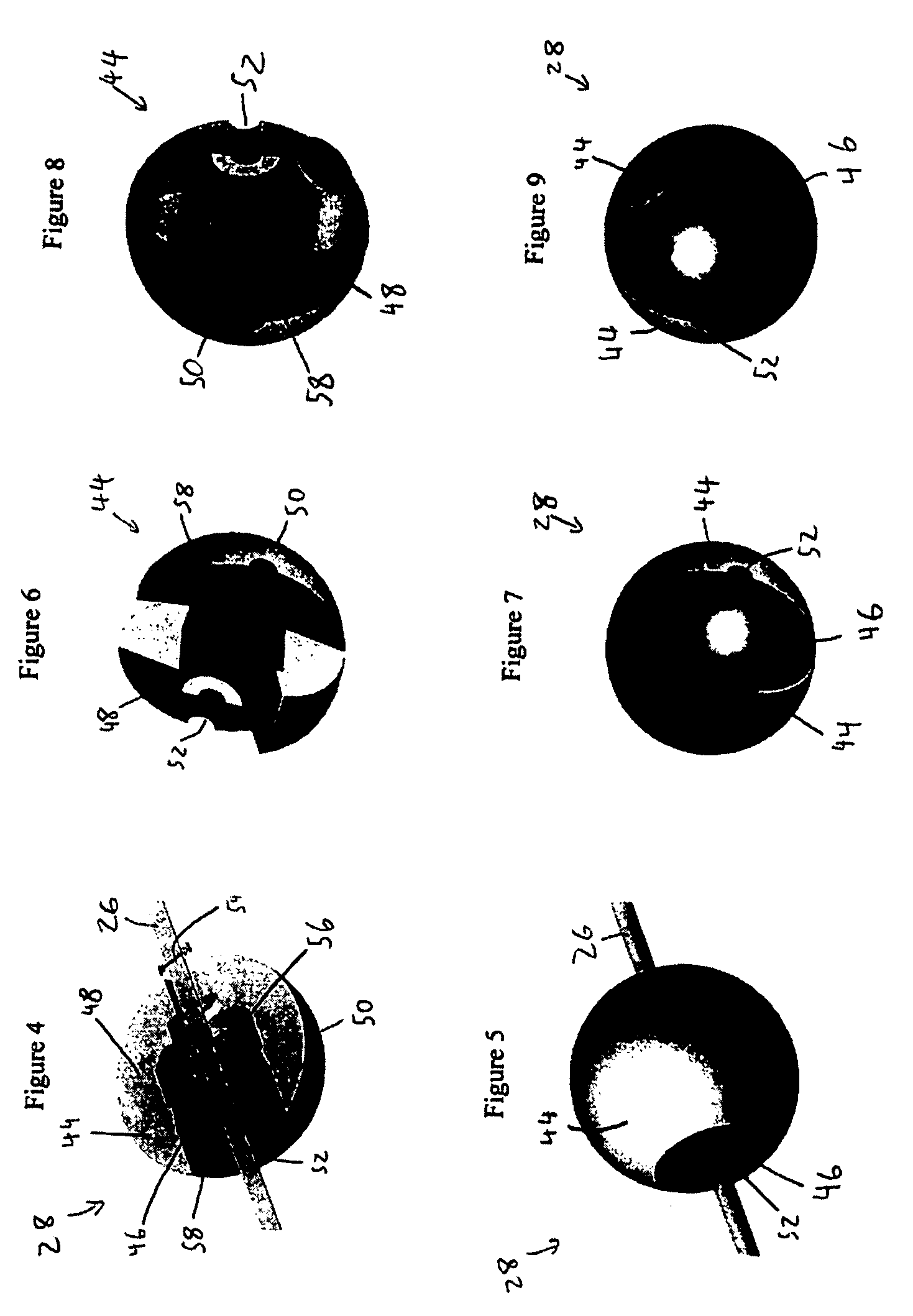
[0051]FIG. 3a illustrates an embodiment of a vascular embolization or occlusion device 24 having a flexible leader 26 that can be connected to a first non-expandable space-occupying element 28 and a second non-expandable space-occupying element 30. Additional non-expandable space-occupying elements 32 can also be connected to the leader 26 and provided in various lengths, depending on the typical volume of the sac 10 to be filled. The leader 26 can pass through the elements 28, 30 and 32. The leader 26 can be fixed to the elements 28, 30 and 32, or the elements 28, 30 and 32 can slide freely over the leader 26. As illustrated in FIG. 3b, the leader 26, even if secured within an element 28, 30, or 32, can flex and bend within each element 28, 30 or 32, or between the elements 28, 30 and 32.
[0052] The leader 26 can be a suture, preformed resilient structure, poppet, wire, fiber, monofilament, rail, or a woven thread or other combination thereof. The leader 26 can be completely separa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com