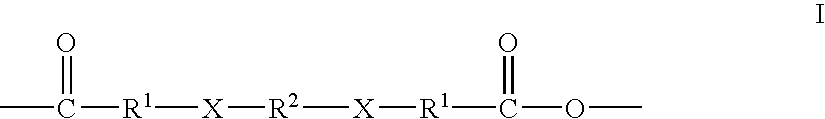Therapeutic polyanhydride compounds for drug delivery
a technology of polyanhydride compounds and drug delivery, applied in the direction of drugs, prostheses, immunological disorders, etc., can solve the problems of slow degradation time, relatively insoluble degradation products, difficult processing, etc., and achieve the effect of improving solubility and processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Amoxicillin Polymer
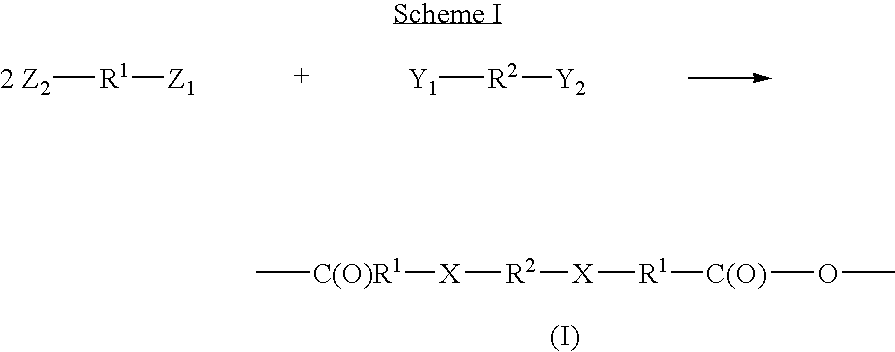
The linkage of amoxicillin in a polyanhydride of the present invention is shown in the scheme 1. The carboxylic acid group of the low molecular weight drug molecule is protected, preferably via acetylation. The protected drug molecules are then exposed to a chlorinated form of the linker of formula (IV), wherein n is 8. The amine groups from the drug molecules displace the chlorine groups of the diacyl halide Formula (IV) and bind to the linker(R2) via the amine, groups of the drug molecules. The linked drug is exposed to heat and / or vacuum to remove the protecting groups, thereby resulting in a polymeric drug delivery system.
example 2
Synthesis of Cephalexin Polymer
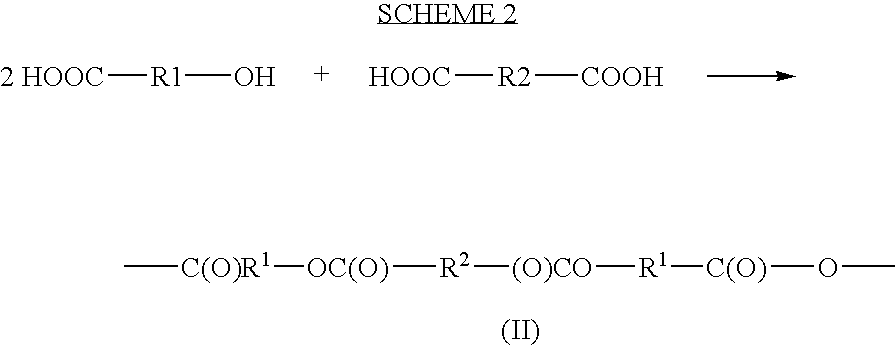
A cephalexin polymer is prepared as depicted in scheme 2. The carboxylic acid group of cephalexin is first protected, for example with a benzylic group. The drug is then linked to sebacoyl chloride (formula (IV) where n is 8). Following this linkage, the protecting groups are removed to produce carboxylic acids which are then acetylated to produce monomer. The monomer is polymerized as a melt.
example 3
Other polymeric drug delivery systems can be prepared in accordance with this method via the polyanhydride linker of Formula (I) of the present invention include, but are certainly not limited to, a carbidopa delivery system, a levodopa delivery system and an amtenac delivery system. Homopolymers of the carbidopa and levodopa drug delivery systems are depicted in Formulas (V) and (VI), respectively
While these structures depict homopolymers, copolymers of such drugs can also be prepared routinely based upon the teachings provided herein. Further, polymeric drug delivery systems comprising the polyanhydride of Formula (I) and other drugs meeting the structural requirements, namely one carboxylic acid group, at least one amine, thiol, alcohol or phenol group, and having a molecular weight of approximately 1000 daltons or less can also be routinely prepared via the disclosed methods.
Activity
The ability of a polymer of the invention to produce a given therapeutic effect can be de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| biocompatible | aaaaa | aaaaa |
| thermal | aaaaa | aaaaa |
| hydrolytic stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com