Novel modified nucleic acid sequences and methods for increasing mRNA levels and protein expression in cell systems
a cell system and protein technology, applied in the field of heterologous gene expression, can solve the problems of difficult recombinant production of certain heterologous gene products, difficult to express proteins derived from bacteria, parasites and viruses in cell culture systems different from the cell from which the protein was originally derived, and serious malaria problems, and achieve the effect of enhancing the expression of sufficient quantities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0048] Creation of Novel Modified MSP-142 Gene
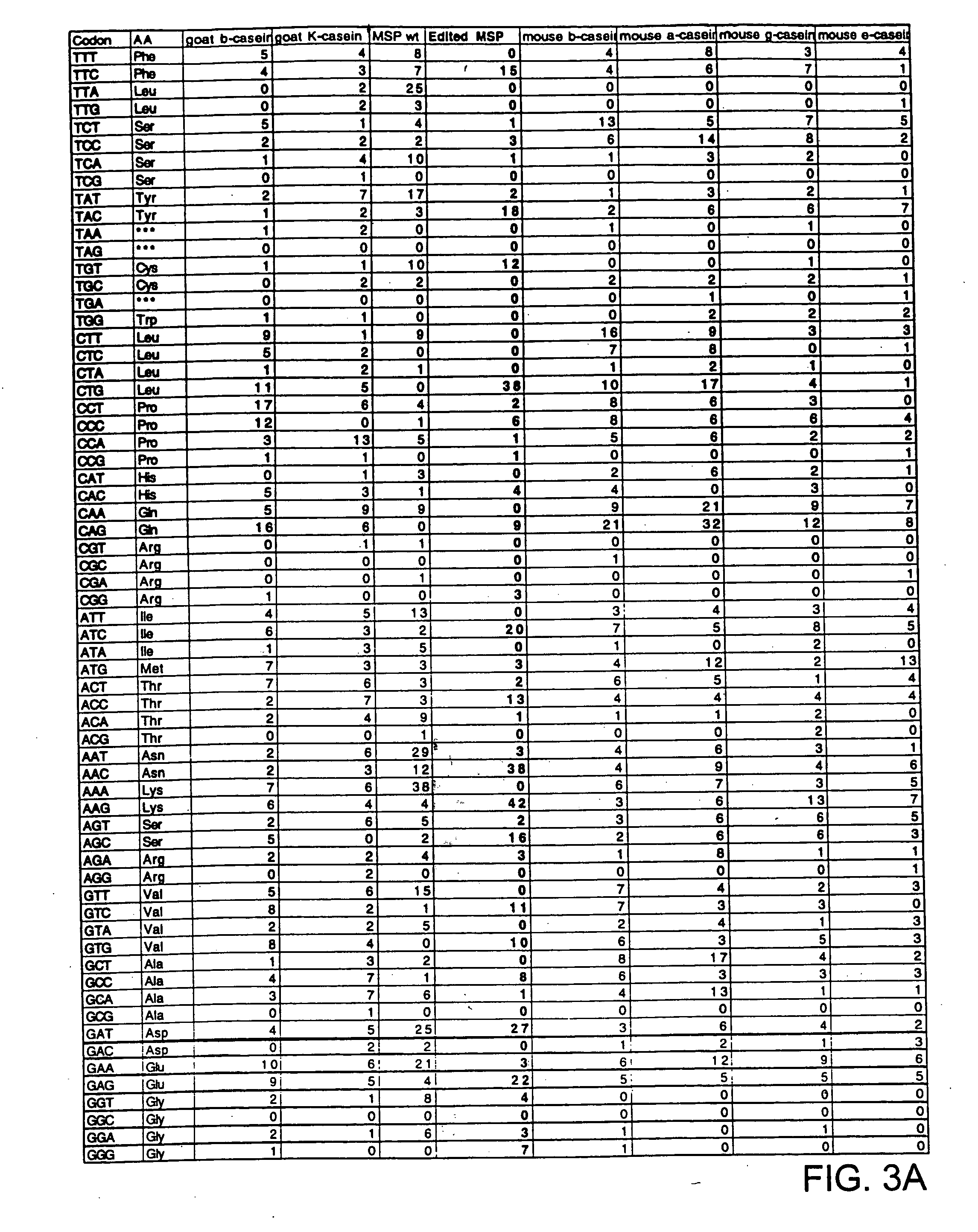
[0049] In one embodiment, a novel modified nucleic acid encoding the C-terminal fragment of MSP-1 is provided. The novel, modified nucleic acid of the invention encoding a 42 kD C-terminal part of MSP-1 (MSP-142) capable of expression in mammalian cells of the invention is shown in FIG. 1. The natural MSP-142 gene (FIG. 2) was not capable of being expressed in mammalian cell culture or in transgenic mice Analysis of the natural MSP-142 gene suggested several characteristics that distinguish it from mammalian genes. First, it has a very high overall AT content of 76%. Second, the mRNA instability motif, AUUUA, occurred 10 times in this 1100 bp DNA segment (FIG. 2). To address these differences a new MSP-142 gene was designed. Silent nucleotide substitution was introduced into the native MSP-142 gene at 306 positions to reduce the overall AT content to 49.7%. Each of the 10 AUUUA mRNA instability motifs in the natural gene were eliminated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic strength | aaaaa | aaaaa |
| ionic strength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com