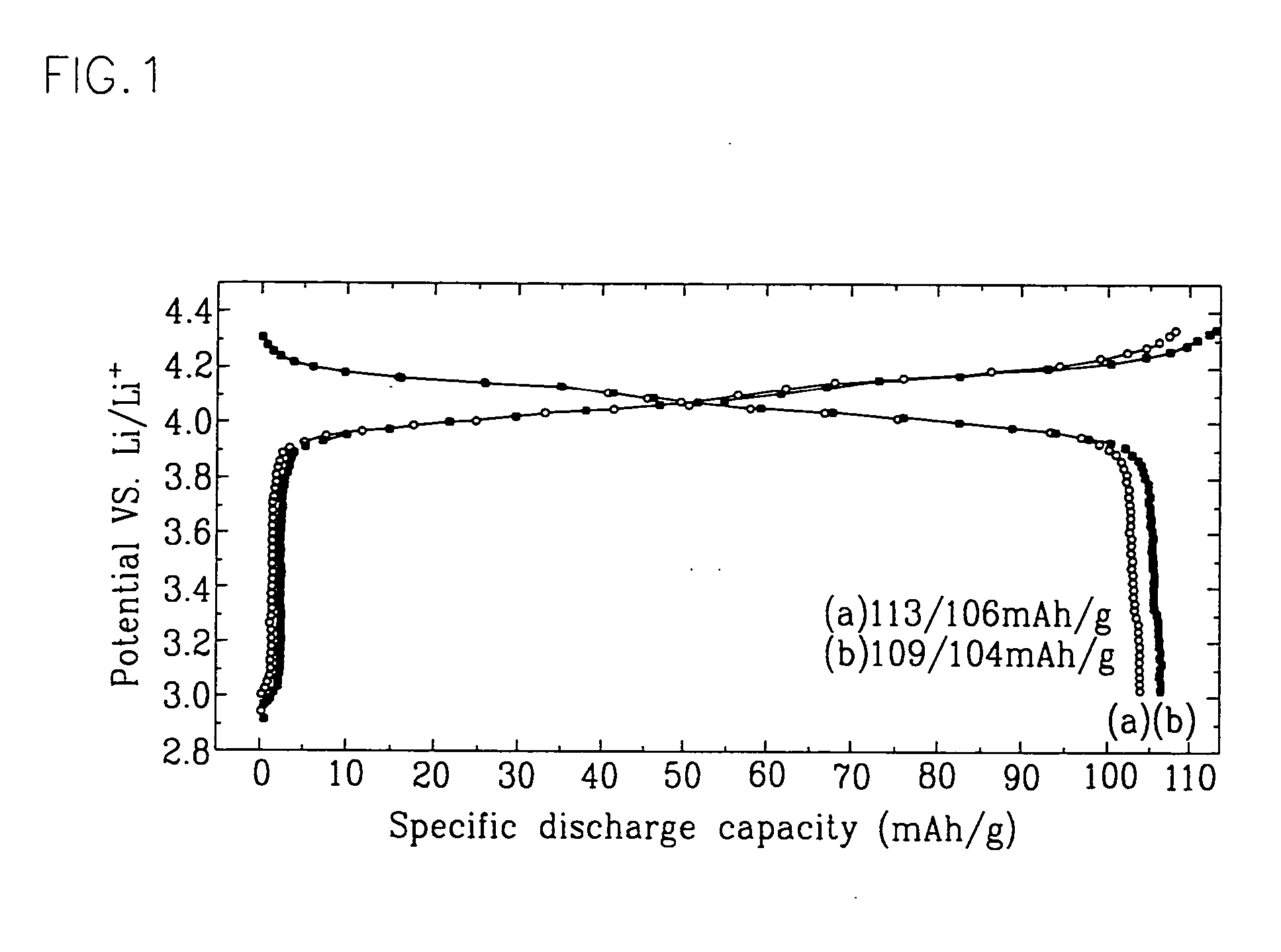
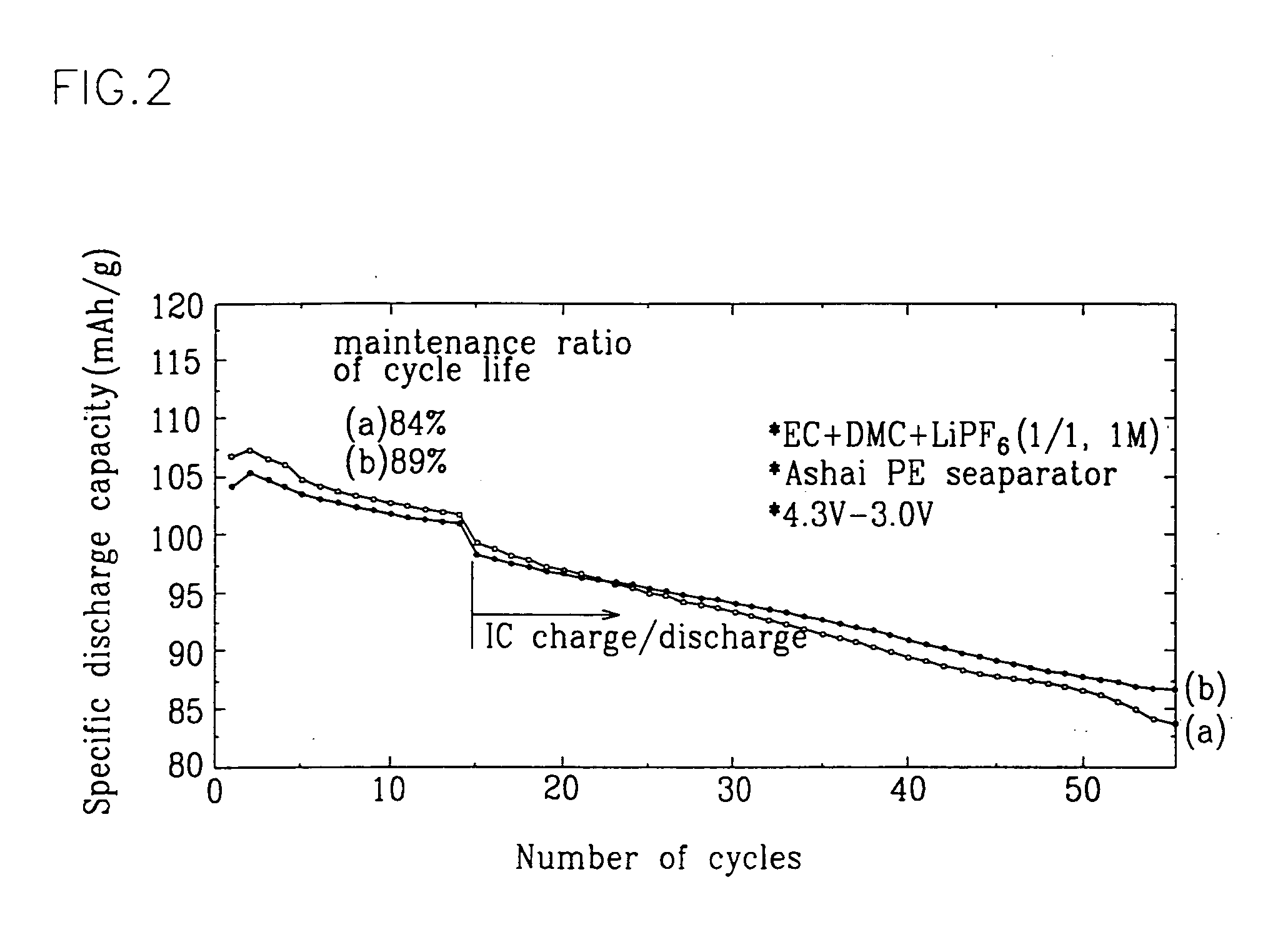
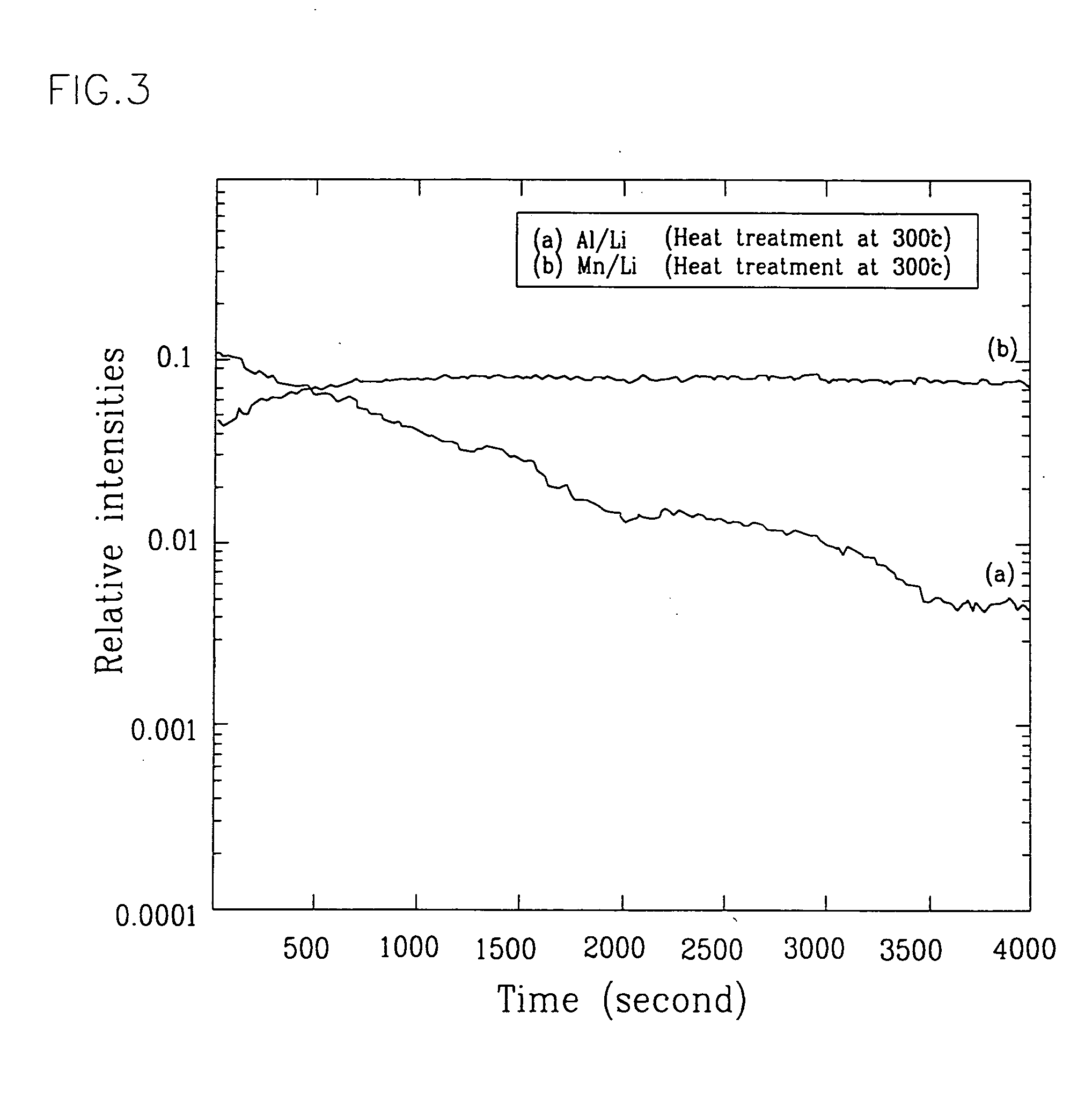
Positive active material for rechargeable lithium battery and method of preparing same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0018] An aluminum isopropoxide solution having a 5 weight-percent concentration was prepared by refluxing an aluminum isopropoxide powder in ethanol at about 100° C. for about half an hour. The aluminum isopropoxide solution was then mixed with a powder of LixMn2-yAlyO4-zFz where 06 for a solute.
example 2
[0019] The positive electrode preparing procedure was performed in the same way as in Example 1 with the exception that the heat-treating temperature was heightened up to 900° C. A coin-type half cell was fabricated with the resulting positive electrode in combination with other components as described in Example 1.
example 3
[0020] An aluminum isopropoxide solution having a 5 weight-percent concentration was prepared by refluxing an aluminum isopropoxide powder in ethanol at about 100° C. for about half an hour. The aluminum isopropoxide solution was then mixed with a powder of LixMn2O4 where 0<x≦1.5 at an identical volume ratio in a moisture free dry room such that an overall surface of the power became wet sufficiently by the solution, and dried in the same room. Thereafter, the mixture was heat-treated at about 300° C. for about 10 hours under a dry air atmosphere to thereby prepare a metallic oxide-coated active material. The subsequent positive electrode processing steps were performed in the same way as in Example 1. A coin-type half cell was fabricated with the resulting positive electrode in combination with other components as described in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com