Method of purification of polymeric medical device materials using continuous soxhlet extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Static Solvent Extraction at Ambient Temperature
[0024] Ten (10) intraocular lenses with a dry weight of 0.3231 g, were submerged and settled on the bottom of a flask filled with 180 cc of isopropanol (IPA). After 3 hours, all lenses were recovered and dried in vacuum oven at 70° C. overnight. The weight of the dried lenses was 0.3144 g, for a loss of 2.69 percent.
example 2
Batch Soxhlet Extraction with Lens Samples in Teflon™ Holder
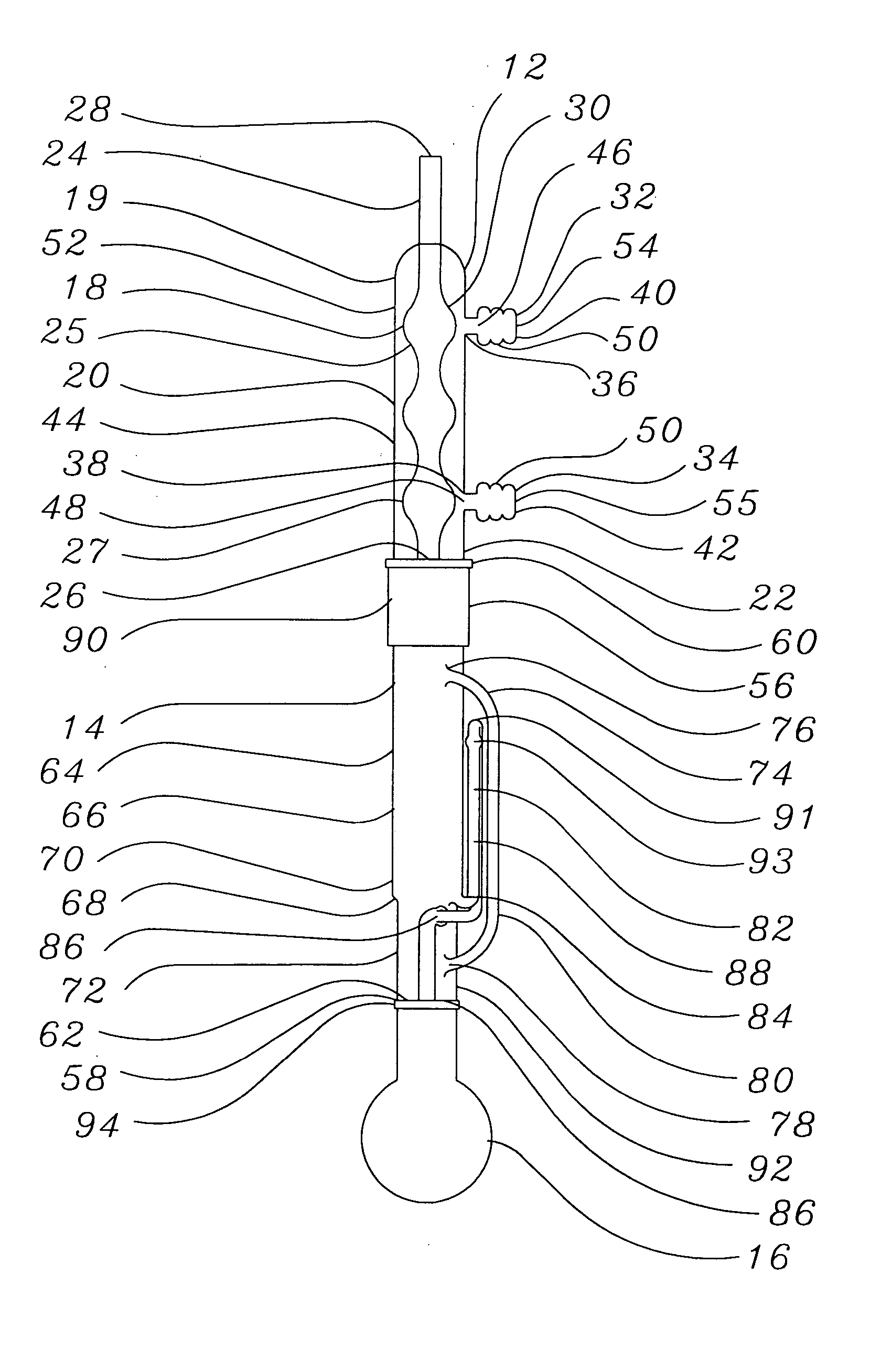
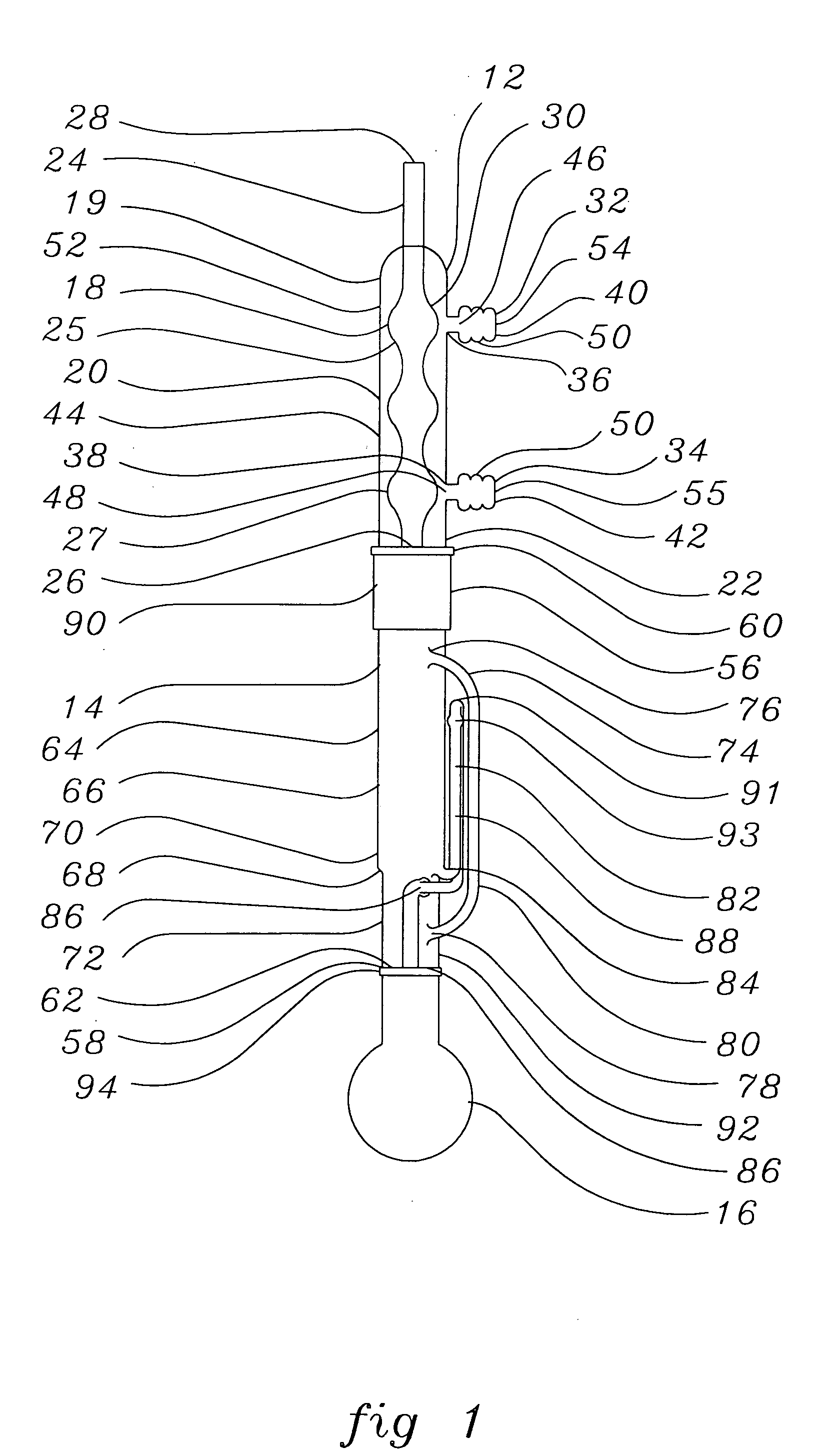
[0025] A soxhlet extractor capable of holding 180 cc of solvent without overflow was attached to a 500 mL round bottom flask filled with IPA and a refluxed condenser. The variance was adjusted and the IPA was heated to reflux. The temperature of the main body of the soxhlet extractor was found to be 75° C.
[0026] Ten (10) intraocular lenses with a dry weight of 0.3223 g were placed in open cages cut out from circular Teflon™ (E.I. Dupont de Nemours, Wilmington, Del.) plates (5 cages on each plate). The Teflon™ plates were then stacked vertically and held together using a central holder. The holder with the plates and lenses was then placed in the soxhlet extractor and underwent extraction for three hours. During the extraction, all solvent siphoned back into the flask once the level of the solvent within the extractor reached a level above that of the peak of the liquid arm. After three hours of extraction, the lenses were...
example 3
Batch Soxhlet Extraction with Lens Samples in Glass Thimble with Coarse Sintered Glass Filter
[0027] Ten (10) intraocular lenses having a dry weight of 0.3216 g were placed in a glass thimble on top of a coarse sintered glass filter placed in the bottom thereof. The glass thimble was then placed in a soxhlet extractor attached to a flask and a condenser. The lenses then underwent extraction for three hours. During the extraction, all solvent except for the solvent within the glass thimble was recycled once the level of the solvent within the extractor reached a level above that of the peak of the liquid arm. Accordingly, the lenses were continuously submerged in solvent throughout the extraction process although solvent flow was not continuous due to recycling. After extraction, the lenses were removed from the glass thimble and air dried for three hours. The lenses were then dried under vacuum at 70° C. overnight. The weight of the dried lenses was 0.3105 g, for a loss of 3.45 perc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Flow rate | aaaaa | aaaaa |
| Hydrophobicity | aaaaa | aaaaa |
| Polymeric | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com