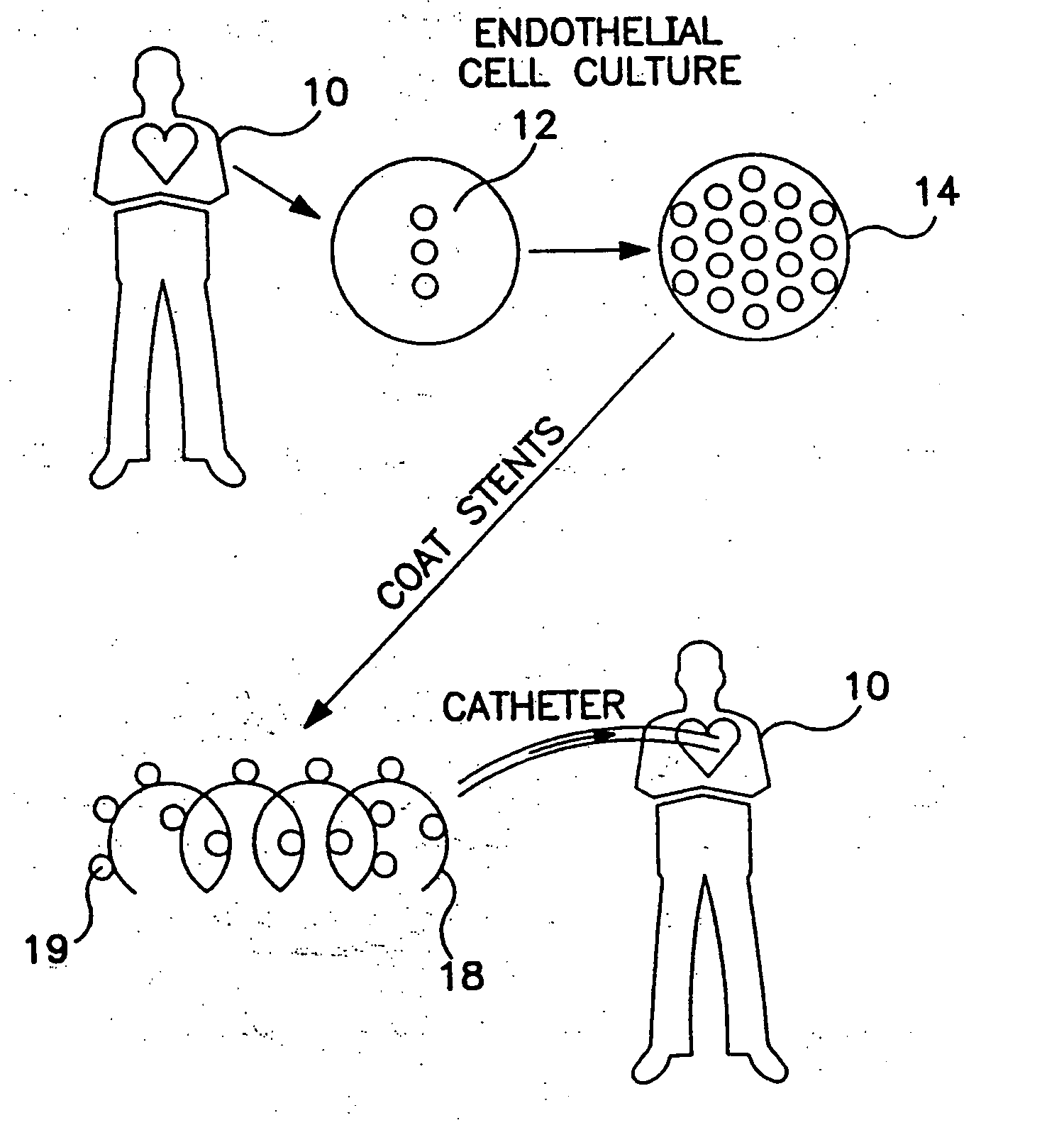
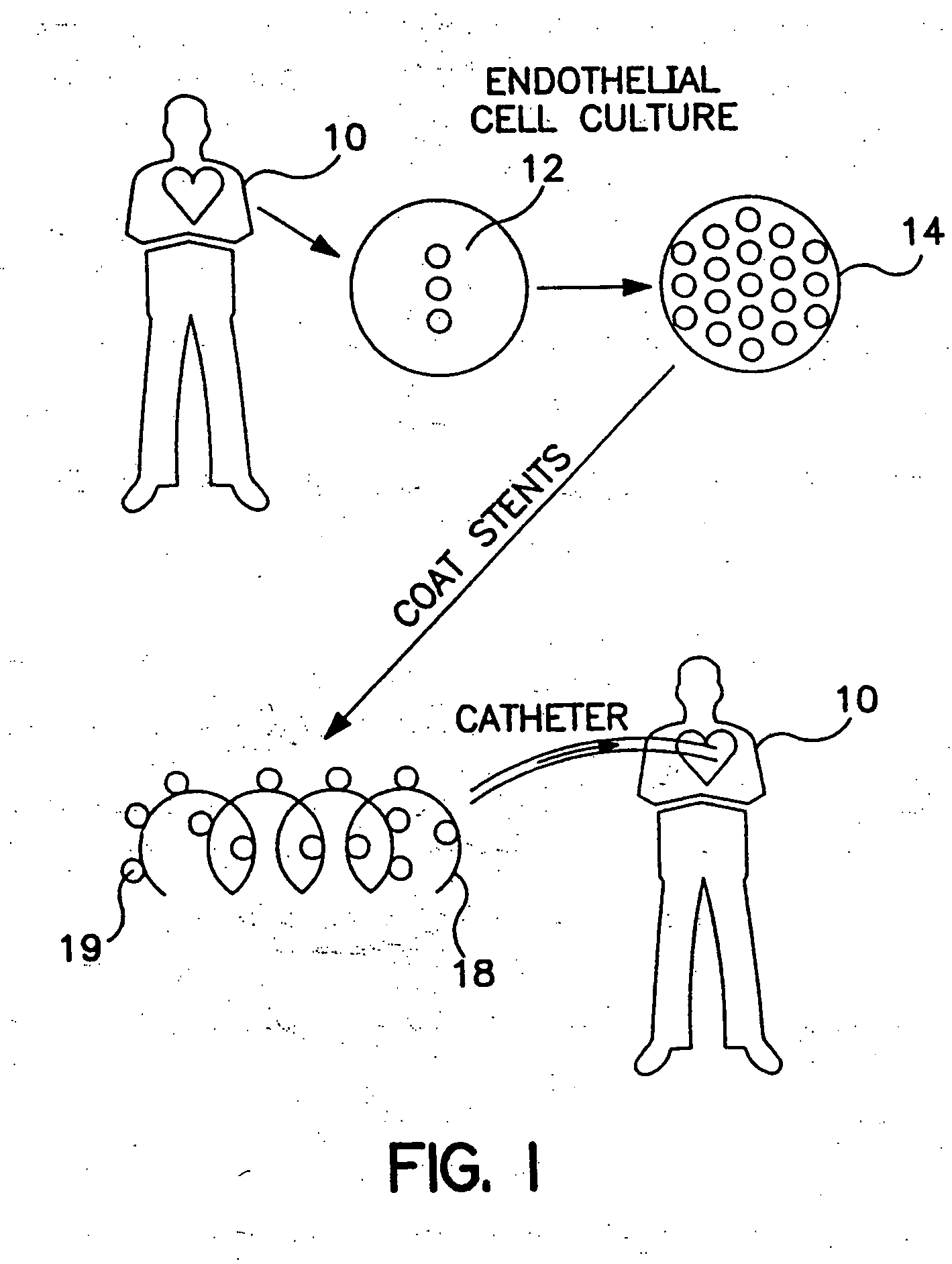
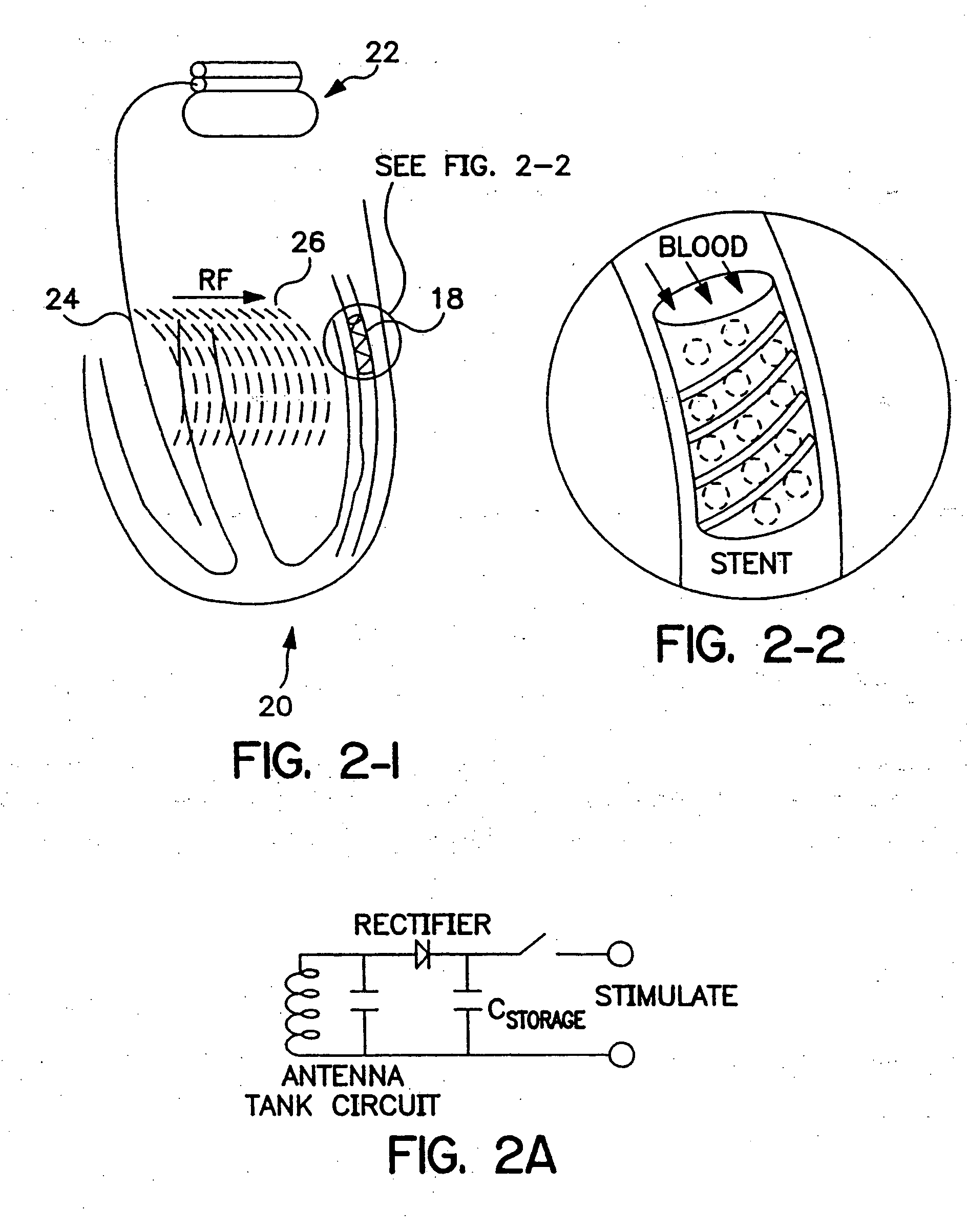
Implantable system with drug-eluting cells for on-demand local drug delivery
a technology of drug-eluting cells and implantable systems, which is applied in the field of implantable systems, can solve problems such as systemic administration and systemic administration can be deleterious, and reduce the potency of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
— example 1
Cell Culture Studies—Example 1
[0092] Human coronary artery endothelial cells (Clonetics, San Diego, Calif.) were seeded in 24 well tissue culture plates at 1×104 cells / cm2 (1.5 mL total volume) and grown in defined media as supplied by Clonetics. The cells were incubated at 37° C., 5% CO2 for up to four days. The media was not changed on the cells during the course of the experiment. Supernatants from duplicate wells were withdrawn on days 2, 3, and 4 post-seeding. In addition, cells plus supernatants from a second set of samples were harvested from duplicate wells on days 2, 3, and 4 post-seeding. Samples were stored at −85° C. until assayed. The ELISA assay was performed on the test samples and on controls following the procedure outlined by the manufacturer (American Diagnostica, Inc.) The standard curve was generated using control plasma A, t-PA antigen control plasma set 2 (Product #275, American Diagnostica, Inc.). The test samples were assayed undiluted (20 μL assay volume). ...
— example 2
Cell Culture Studies—Example 2
[0093] This experiment was performed similarly to the first experiment with the following changes. In the first part of the experiment, cells were seeded at three different densities (1×104, 2×104, 4×104) in 0.5 mL total volume per well, and the cell supernatants were collected on day 2 and day 3 post seeding. In the second part of the experiment, cells were seeded at 2×104 cells / cm2 and one group was stimulated once (on day 2 post seeding) and a second group was stimulated two times (once on day 2 and once on day 3 post seeding). The stimulation level was 55V (350-440 mA) for 5 minutes and supernatants were collected 15 minutes after stimulation. The following tables contain the data that was obtained from this experiment. The data confirms what was observed in the first experiment—that t-PA production / release is correlated with cell number and with cell growth. Although this data from the stimulated samples suggests that electrical stimulation at 55 v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com