Chemical compounds containing a superoxide scavenger and an organic nitrate or nitrite moiety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Antioxidant Nitrate Ester
Step 1
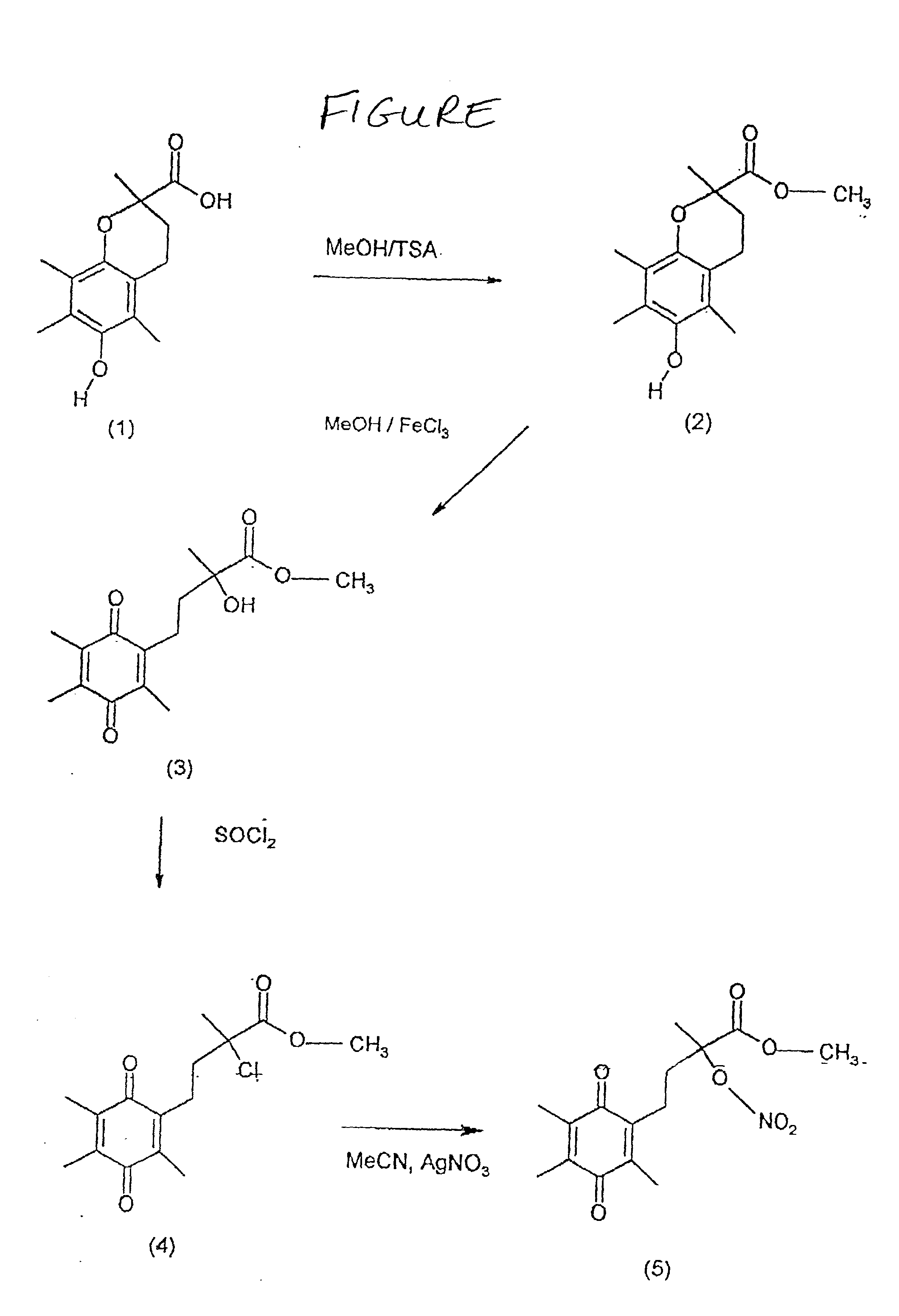
[0034] A solution of 2 g (8 mmol) of optically pure (R)-(+)-3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-carboxylic acid (1) and 0.1 g of p-toluenesulphonic acid monohydrate in 40 ml methanol were stirred and re-fluxed for 4 hours. After cooling, the solution was diluted with water, extracted three times into ether that was subsequently washed with brine and aqueous sodium bicarbonate solution. The ether solution was washed, dried with MgSO4 and evaporated to give (R)-(+)-methyl 3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-carboxylate (2).
Step 2:
[0035] To a stirred solution of 1.5 g (5.68 mmol) of (2) in 22 ml ether, was added a solution of 4.5 g (16.6 mmol) of ferric chloridehexahydrate in 17 ml of water and 17 ml of methanol. The addition was carried out drop-wise over 30 minutes. After 1 hour the ether layer was separated, and the aqueous phase was further extracted with ether. The combined ether laye...
example 2
Exemplification of Protective Effect of Antioxidant
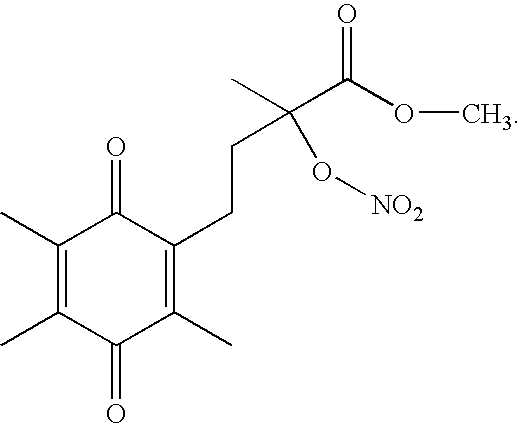
[0038] Buffered solutions containing an organic nitrate as follows [all containing vitamin C between 1 and S equivalents]: (i) propyl nitrate, (ii) propyl nitrate plus one equivalent of compound (3) and (iii) compound (S) in the range of 1 to 10 μmol were prepared. These solutions were incubated in sealed glass vessels with appropriate concentrations of XO and NADH under both hypoxic and normoxic conditions. After 24 hours levels of NO were measured by aspirating the glass vessels and monitoring NO concentration directly. The results demonstrated the ability of superoxide scavengers to minimize superoxide interaction with NO upon activation of organic nitrates.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com