Anti-IGF-I receptor antibody
a technology of anti-igf receptor and antibody, which is applied in the field of antibodies, can solve problems such as agonistic activity, and achieve the effect of increasing utility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
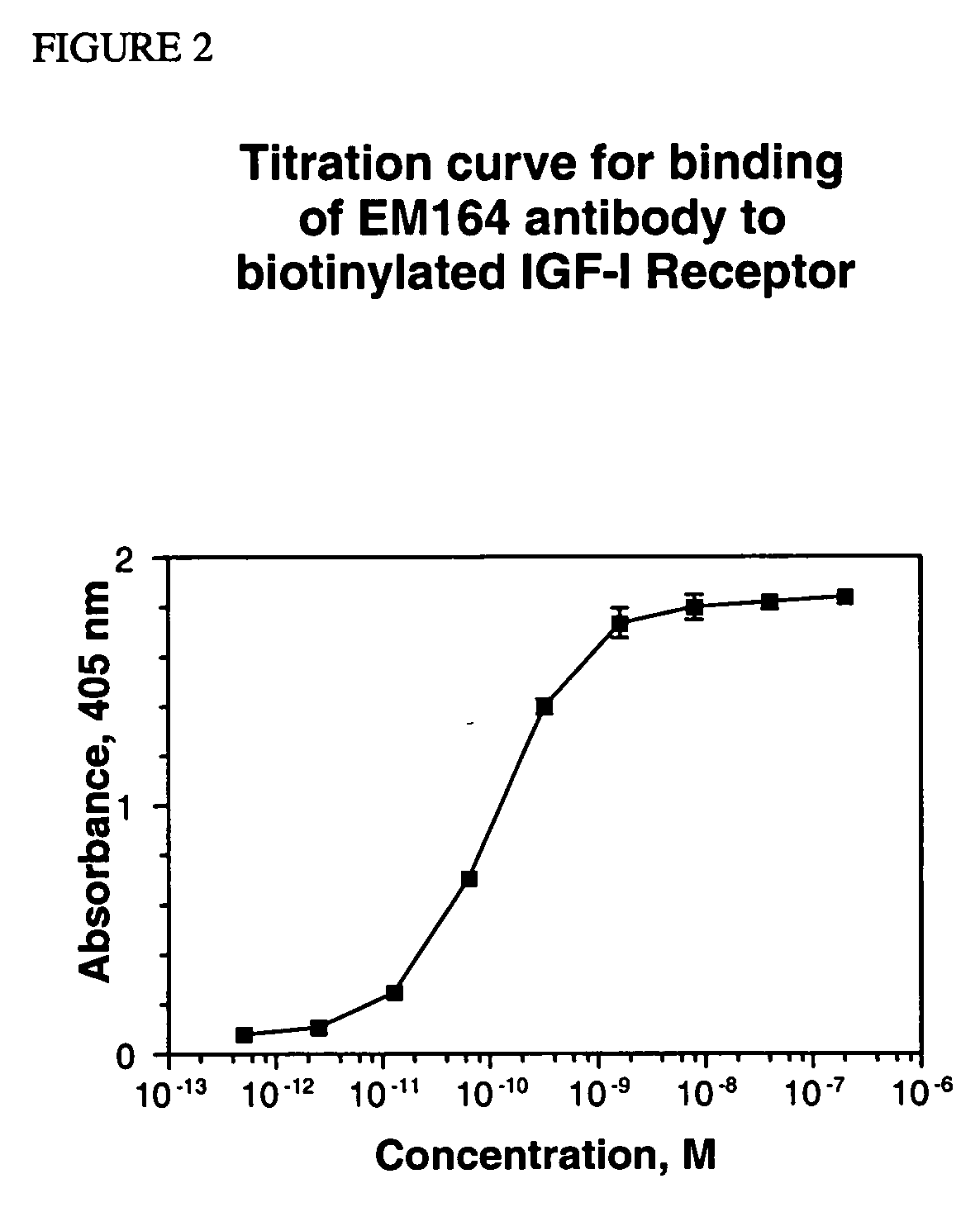
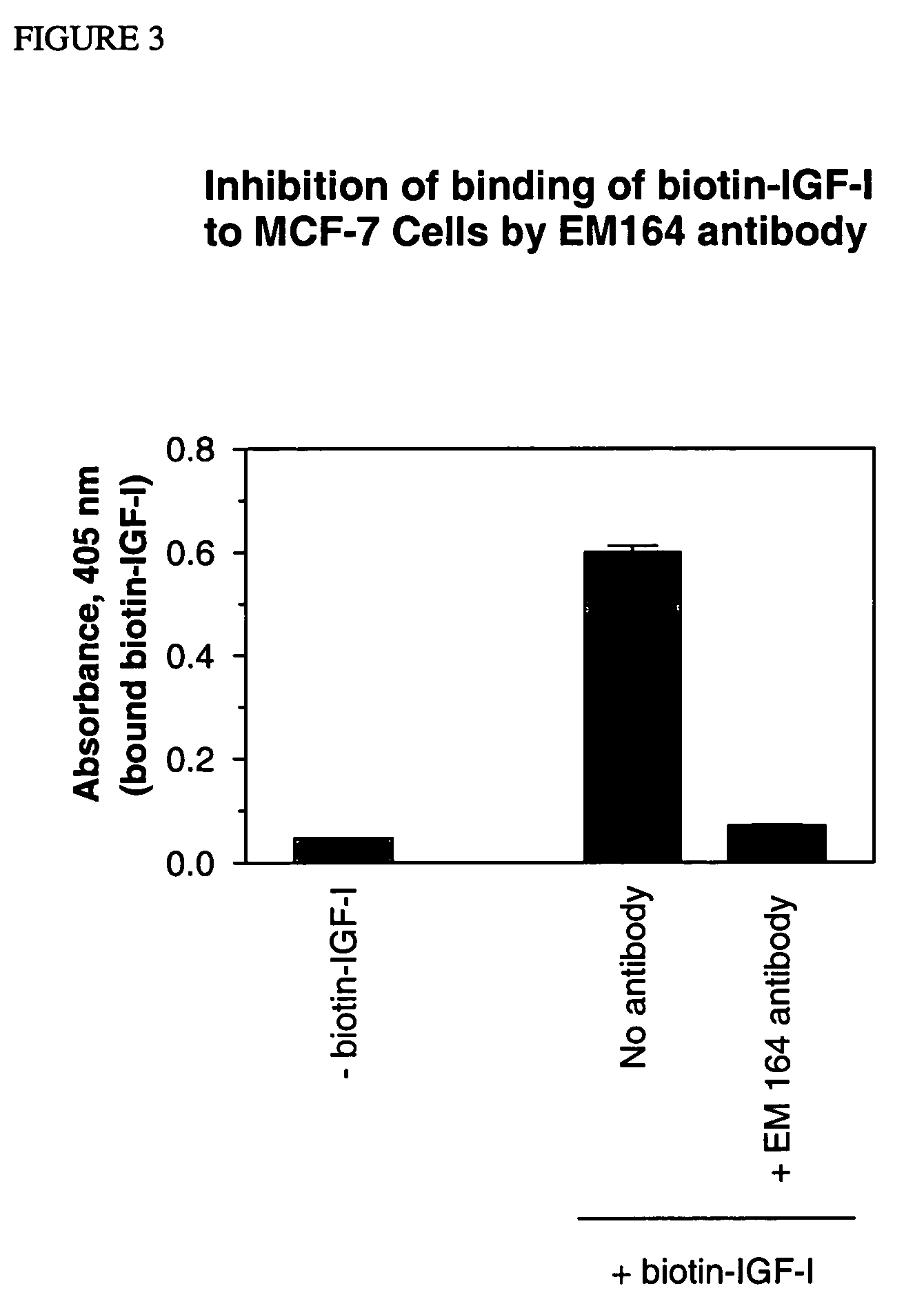
Murine EM164 Antibody
[0106] In this first example, the complete primary amino acid structure and cDNA sequence of a murine antibody of the present invention is disclosed, together with its binding properties and means for its expression in recombinant form. Accordingly, there is provided a full and complete disclosure of an antibody of the invention and its preparation, such that one of ordinary skill in the immunological arts would be able to prepare said antibody without undue experimentation.
A. Generation of Anti-IGF-I Receptor Monoclonal Antibody Hybridoma
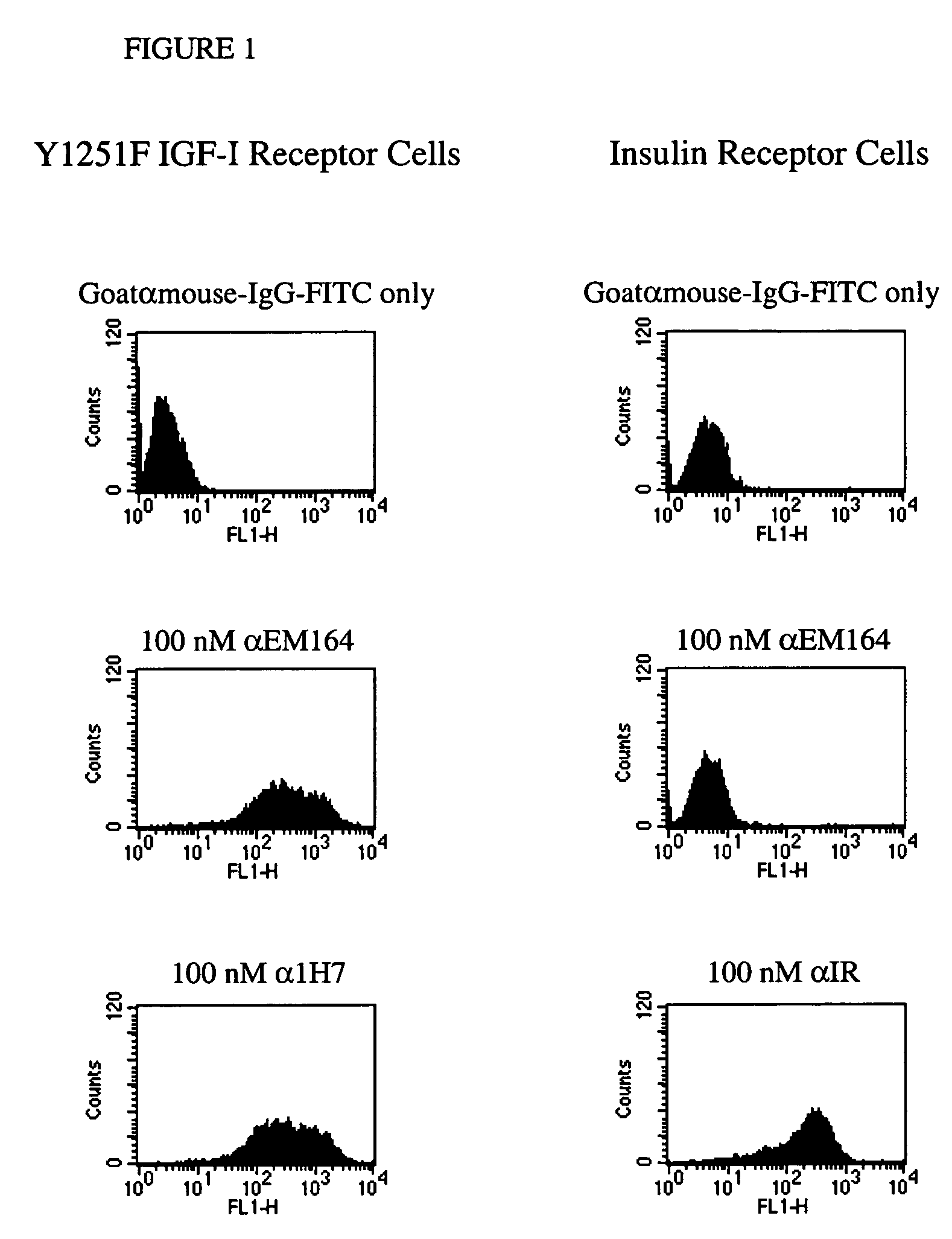
[0107] A cell line expressing human IGF-I receptor with a Y1251F mutation was used for immunization as it expressed a high number of IGF-I receptors (˜107 per cell). The Y1251F-mutation in the cytoplasmic domain of IGF-I receptor resulted in loss of transformation and anti-apoptotic signaling, but did not affect IGF-I binding and IGF-I-stimulated mitogenic signaling (O'Connor, R. et al., 1997, Mol. Cell. Biol., 17, 427-435;...
example 2
Humanized Versions of EM164 Antibody
[0166] Resurfacing of the EM164 antibody to provide humanized versions suitable as therapeutic or diagnostic agents generally proceeds according to the principles and methods disclosed in U.S. Pat. No. 5,639,641, and as follows.
A. Surface Prediction
[0167] The solvent accessibility of the variable region residues for a set of antibodies with solved structures was used to predict the surface residues for the murine anti-IGF-I receptor antibody (EM164) variable region. The amino acid solvent accessibility for a set of 127 unique antibody structure files (Table 2) were calculated with the MC software package (Pedersen et al., 1994, J. Mol. Biol., 235, 959-973). The ten most similar light chain and heavy chain amino acid sequences from this set of 127 structures were determined by sequence alignment. The average solvent accessibility for each variable region residue was calculated, and positions with greater than a 30% average accessibility were co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com