Medicinal compositions containing diuretic and insulin resistance-improving agent
a technology of diuretic and insulin resistance, which is applied in the direction of drug compositions, biocides, metabolic disorders, etc., can solve the problems of increased heart weight, cardiac enlargement, and sometimes adverse events of insulin sensitizers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
Example 1
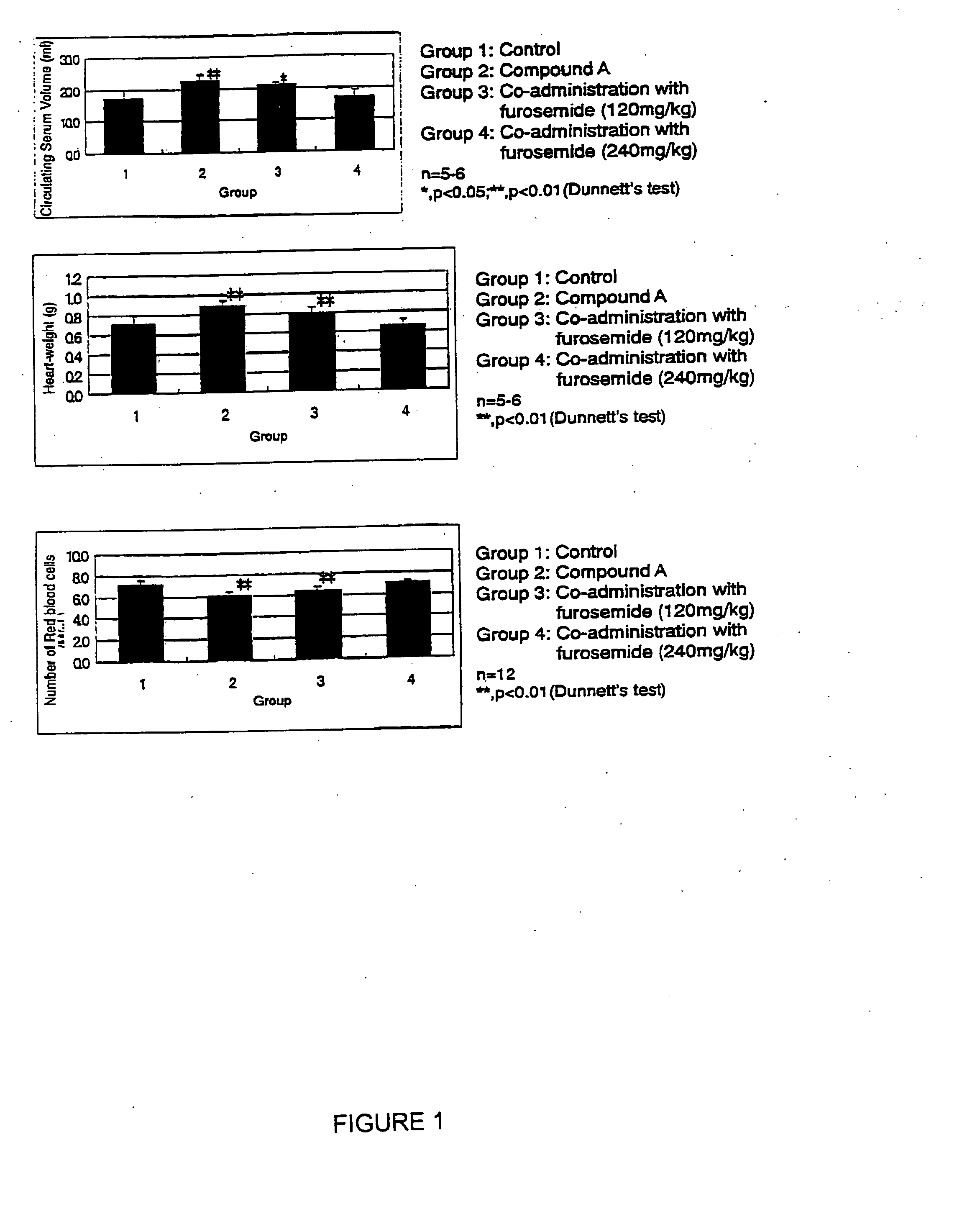
Improving Effects of Co-Administration of a Diuretic, Furosemide, on Increasing Heart-Weight and Edema Elicited by 5-[4-(6-methoxy-1-methyl-1H-benzimidazol -2-ylmethoxy)benzyl]thiazolidine-2,4-dione Hydrochloride (Compound A)
(1) Increases in Heart-Weight and Circulating Serum Volume
[0289] After 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione hydrochloride (200 mg / kg) was repeatedly and orally administered by gavage for 3 or 7 successive days to female Wistar rats (7 weeks old, Charles River Japan, Inc.), circulating serum volume was determined by Evans blue methods and heart-weight recorded. The results are summarized in Table 1.
TABLE 1Duration ofRatio aganist controlAdministrationCirculating(day)Heart-WeightSerum volume31.03a)1.18a)*71.22b)**1.30b)**
Note:
a)n = 5-6;
b)n = 12;
*p < 0.05;
**p < 0.01 (Student's t-test)
[0290] Circulating serum volume and heart-weight were increased after administration for both 3 and 7 days. Therefore ...
example 2
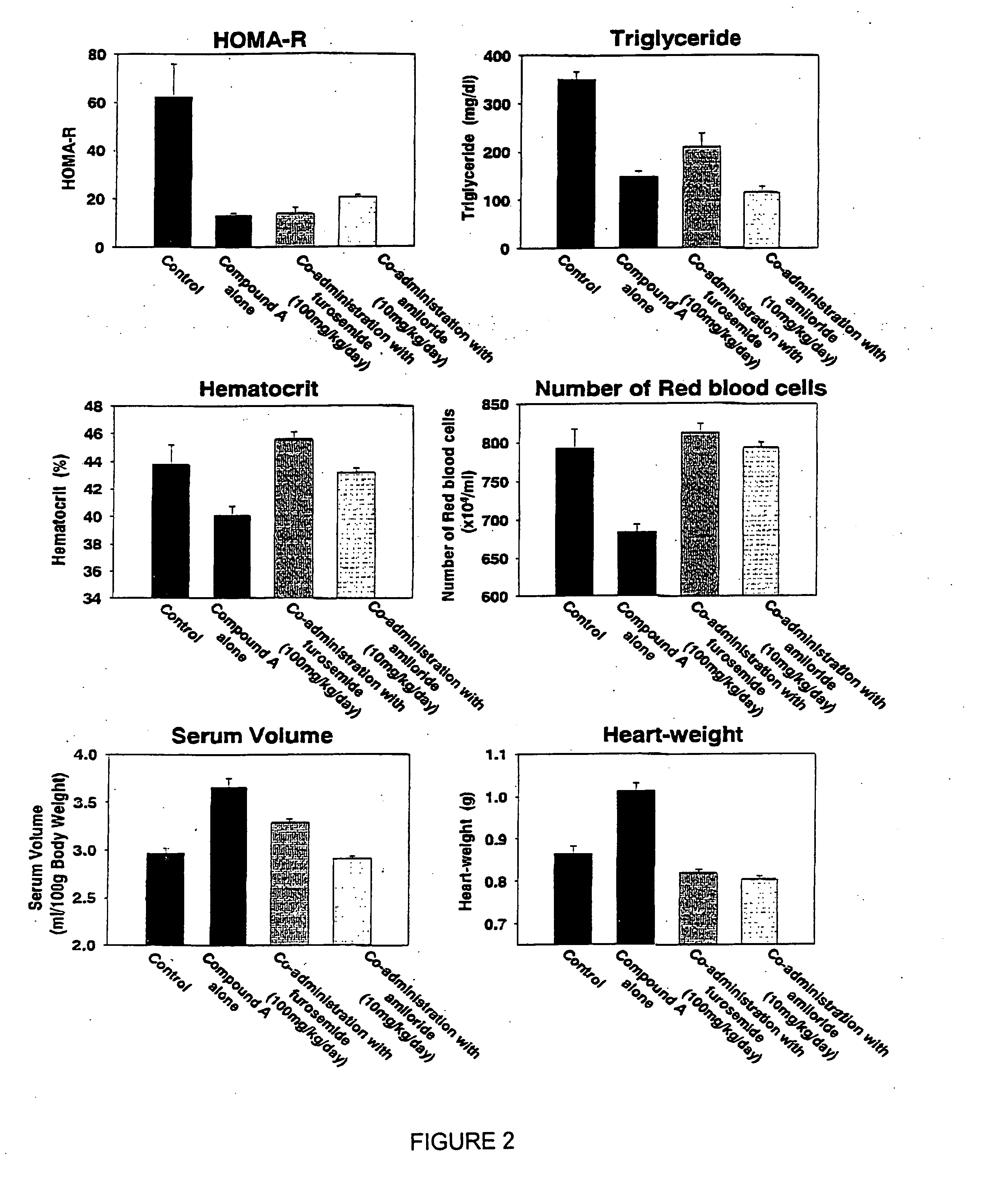
Effects of a Co-Administering a Diuretic, Amiloride, on Increases in Circulating Serum Volume, Heart-Weight, and Edema Caused by Administration of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione Hydrochloride (Compound A)
[0294] Zucker fatty rats (7 weeks old, SLC, Shizuoka, Japan) were grouped as 5 rats a group. Mean body weight, plasma glucose and glyceride levels, hematocrit value, and the number of red blood cells in each group were similar among the groups. The rats were allowed to take food pellets (F2, Funabashi Farm) and water ad libitum. In the control group, the rats were given only food pellets, while the rest of rats were administered 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione hydrochloride (suspended in 0.5% solution of carboxymethylcellulose (CMC)) at a volume of 1 ml / kg orally by gavage. In the groups of rats administered the above compound with furosemide (100 mg / ml, suspended in 0.5% CMC solu...
reference example 1
5-[4-(6-Methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione•hydrochloride
Reference Example 1(1)
5-[4-(6-Methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione
[0300] A reaction mixture of 5-methoxy-N-methyl-1,2-phenylenediamine (21.8 g) (see Reference example 9 of Japanese Patent Application Publication No. Hei 9-295970), 5-(4-methoxycarbonylmethoxybenzyl)thiazolidine-2,4-dione (63.4 g) (see Reference example 21 of Japanese Patent Application Publication No. Hei 9-295970), 1,4-dioxane (250 ml) and concentrated hydrochloric acid (750 ml) was heated under reflux for 60 hours. The reaction mixture was cooled in an ice bath, and the resulting precipitate was filtered off. To this precipitate was added 5% aqueous sodium hydrogencarbonate solution (800 ml), and the mixture was stirred at room temperature for 2 hours. The precipitate was filtered off, dissolved in a mixed solvent of N,N-dimethylformamide (1000 ml) and methanol (200 ml), and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| heart-weight | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com