Compounds and methods for the treatment of ubiquitin conjugating disorders
a conjugating disorder and compound technology, applied in the field of compound and method for the treatment of ubiquitin conjugating disorders, can solve the problems of inability to selectively target e2, high drug resistance, and high cost, and achieve the effect of inhibiting its activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Ubc Inhibitors by Structure Based Screening
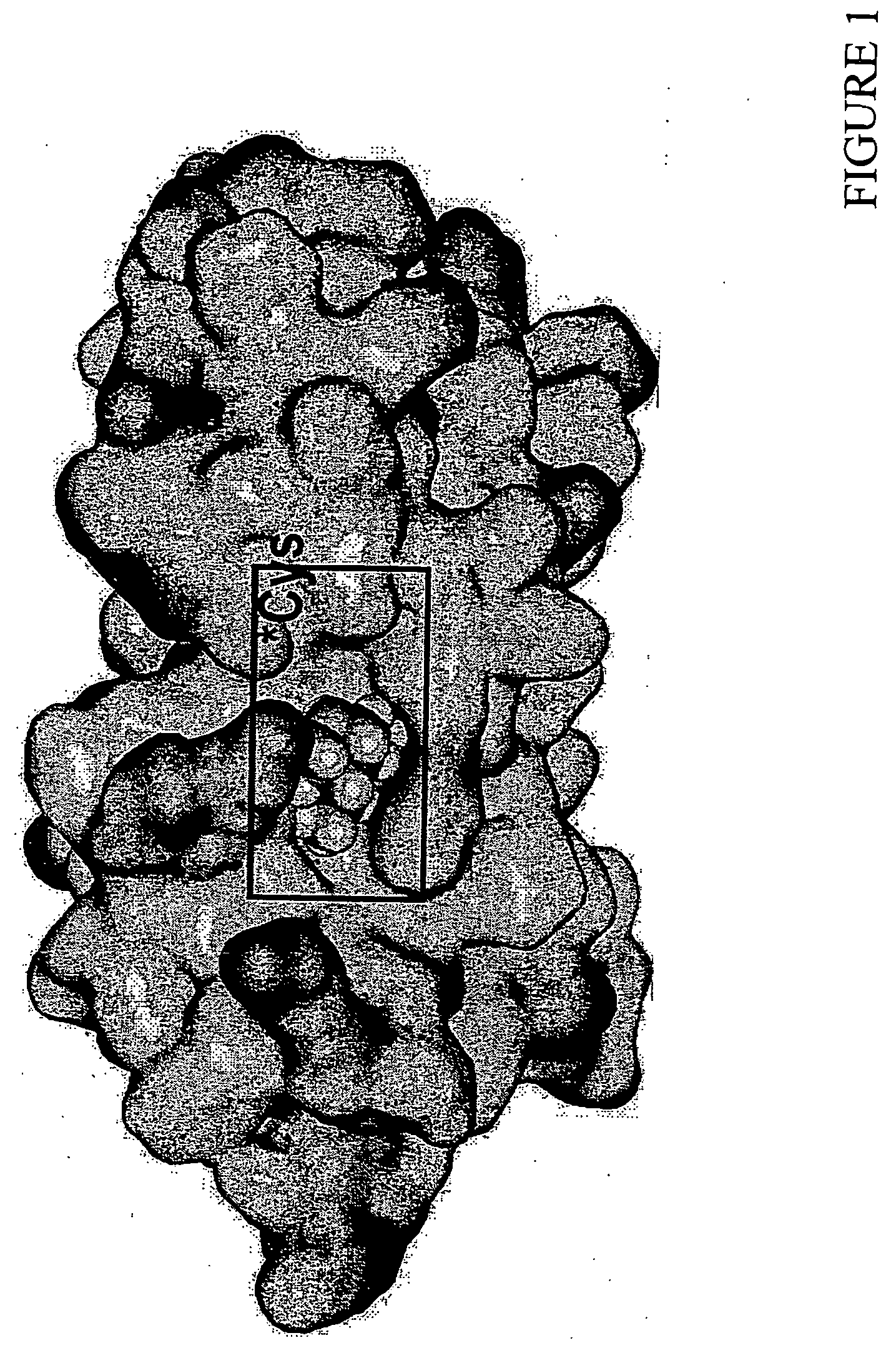
[0191] A structure based screening assay was performed on yeast Ubc4 to identify compounds that bind to the active sites of these molecules. 3-D coordinates for yeast Ubc4 (PDB ID No. 1QCQ) were entered into the InsightII program (InsightII, Accelrys, Inc., San Diego, Calif.). Calculations were performed on the molecule to identify the active sites. Yeast Ubc4 active sites identified with the InsightII program included amino acids Lys64, Pro66, Lys67, Ile68, Asn84, Ile85, Leu90, Lys91 and Leu120. Similarly, another identified active site included amino acids Pro65, Pro66, Lys67, Ile68, Asn84, Leu90, Lys91 and Leu120 of S. cervisiae Ubc4. Active sites in human Ubc5a included Lys66, Ile67, Ala68, Ser83, Cys85, Leu86, Leu89 and Arg90 of SEQ ID NO:2 and Pro64, Pro65, Lys66, Ile67, Ser83, Ile84, Cys85, Leu86, Leu89, Arg90 and Leu119 of SEQ ID NO:2. Active sites in S. cervisiae Ubc7 included Pro68, Lys70, Tyr83, Glu87, Val88, C...
example 2
Determination of IC50 Values for HBS01 in the Breast Cell Lines MCF-7 and MCF-7-Adr
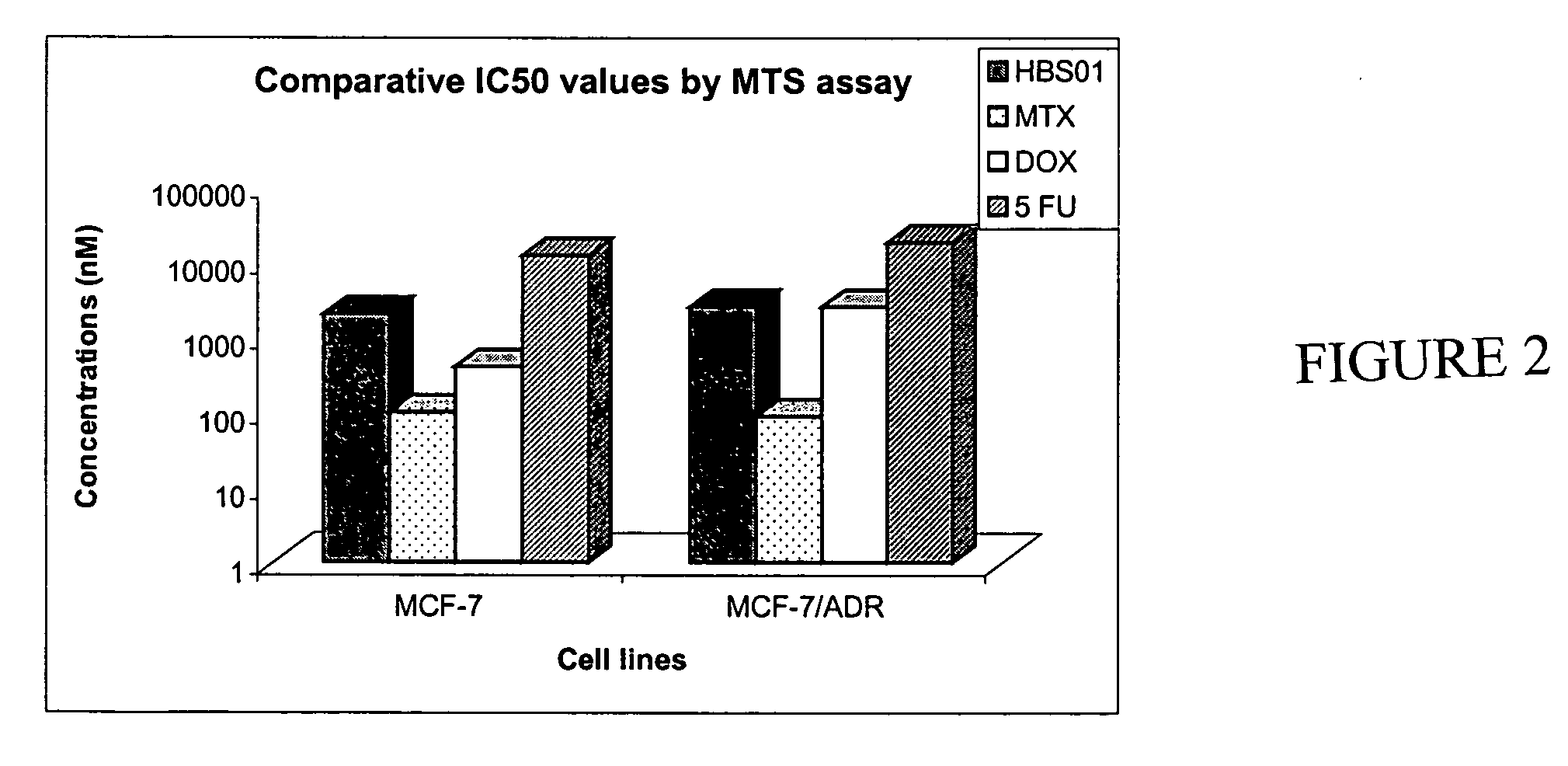
[0194] In order to determine the IC50 values of HBS01 an MTS assay was performed on MCF-7 and MCF-7 / ADR (adramycin resistance) cell lines. Cells were plated in a 96 well plate at 5,000 cells / well. After plating, cells were treated for 96 hours with either HBS01, methotrexate (MTX), doxyrubicin (DOX), or 5-Fluorouracil (5FU) and an MTS assay was performed according to manufacturer's protocol with the CELL TITER 96 AQ non-radioactive cell proliferation assay kit (Promega, Cat. No. G5421). Viable cells were quantitated based on absorbance at 490 nm. IC50 values for HBS01, MTX, DOX and 5FU are shown in FIG. 2. The results indicated that HBS01 had an IC50 value of 30 nM in MCF-7 and MCF-7Adr. Thus, HBS01 was an extremely potent inhibitor of MCF-7 and MCF-7Adr proliferation.
example 3
Effect of HBS01 in C85 Colorectal Tumors in Mice
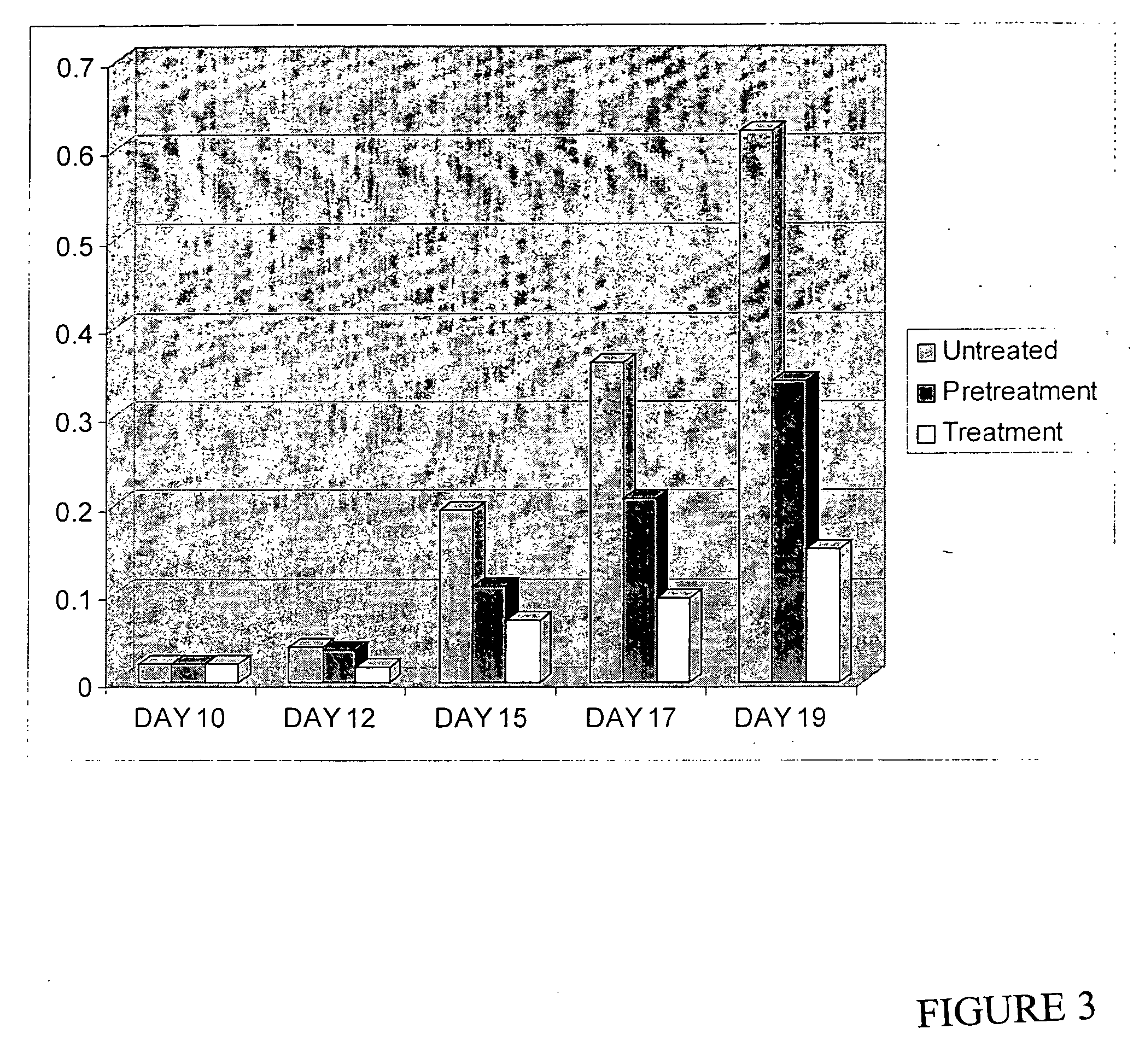
[0195] C85 human colorectal cancer cell growth was assessed in nude mice (NIH, Cr:(MCr)-Fox1nu(nu / nu homogygous), Taconic Inc., Germantown, N.Y.) treated or pretreated with HBS01 over a 19 day period. Briefly, pretreated animals were administered three daily doses of HBS01 at 17 mg / Kg given on day −3, −2, and −1 prior to tumor cell injection on day 0. No further treatment was given to the pretreated animals. Nude mice were injected in the flanks with 1 million C85 human colorectal cancer cells. Treated animals were treated with daily injections of HBS01 at 17 mg / kg per at days 10, 11, 12, 15, 16 and 17 following appearance of palpable tumors on day 10.
[0196] Tumor diameters were measured with calipers every alternate day and tumor volumes were calculated by ½ab2 where a is the larger diameter (Friedman, HS., et al. Cancer Res. 46,2827-2833). The results are shown in FIG. 3 as tumor volume in cm3. The results indicated that treatment ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com