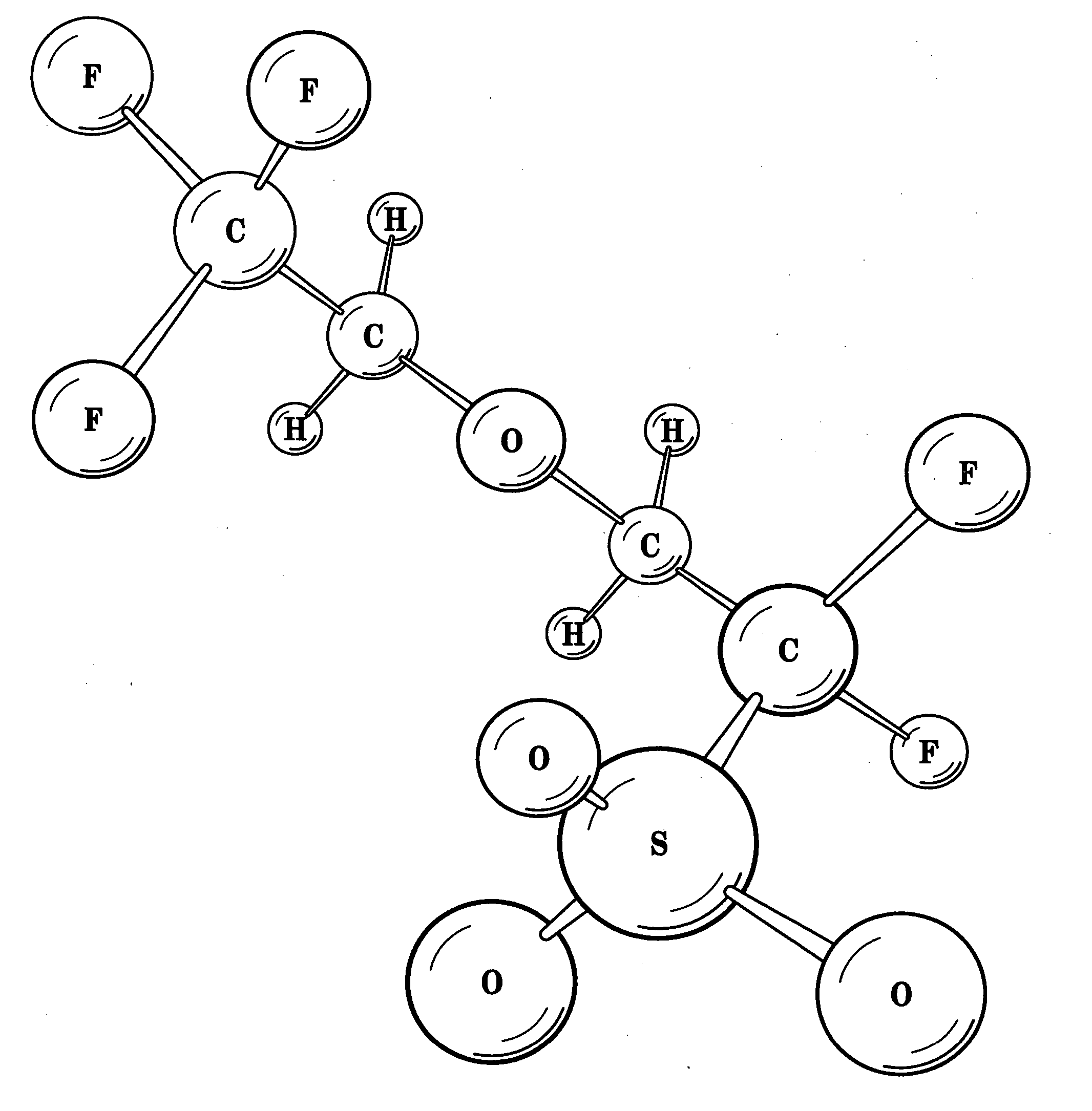
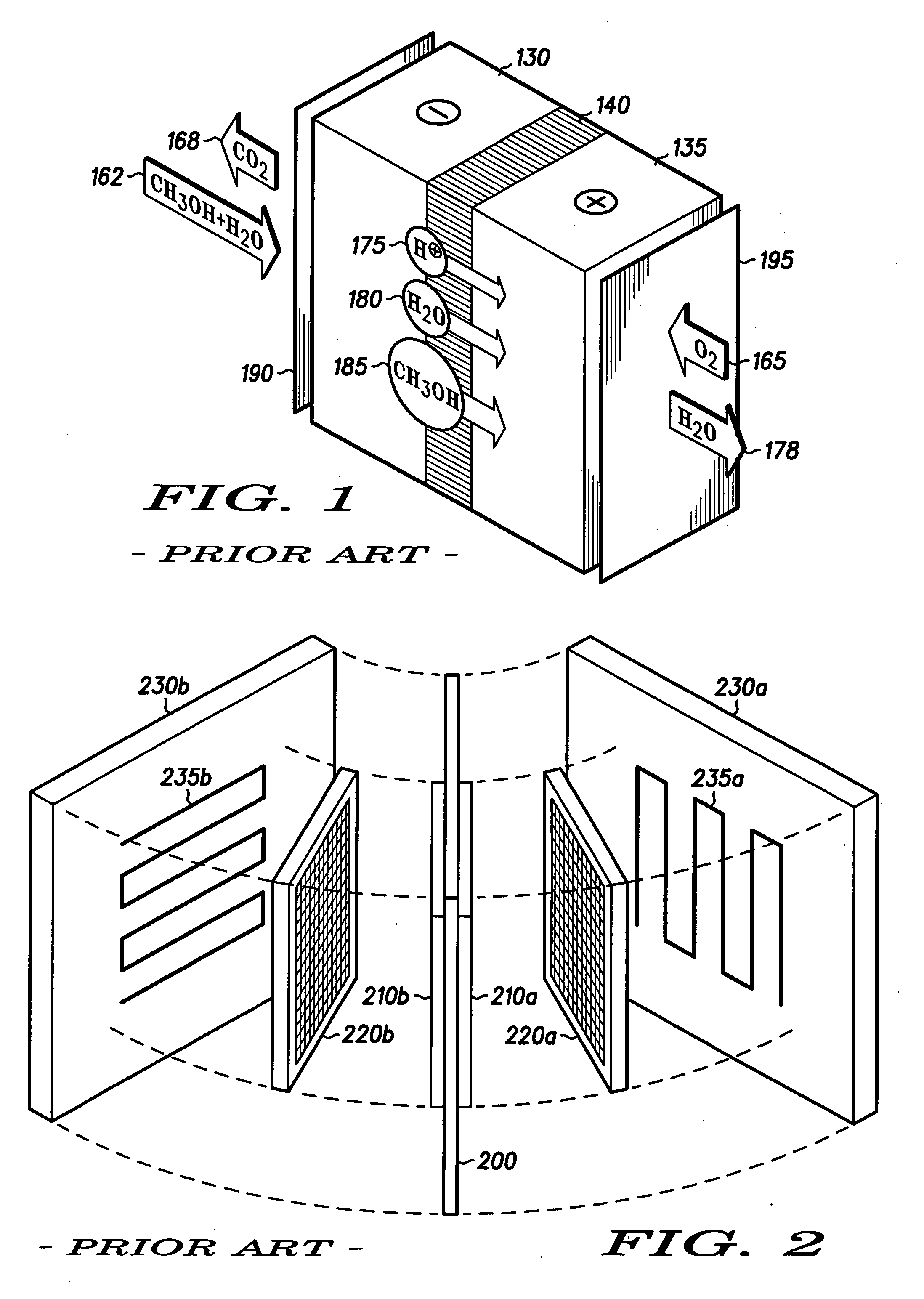
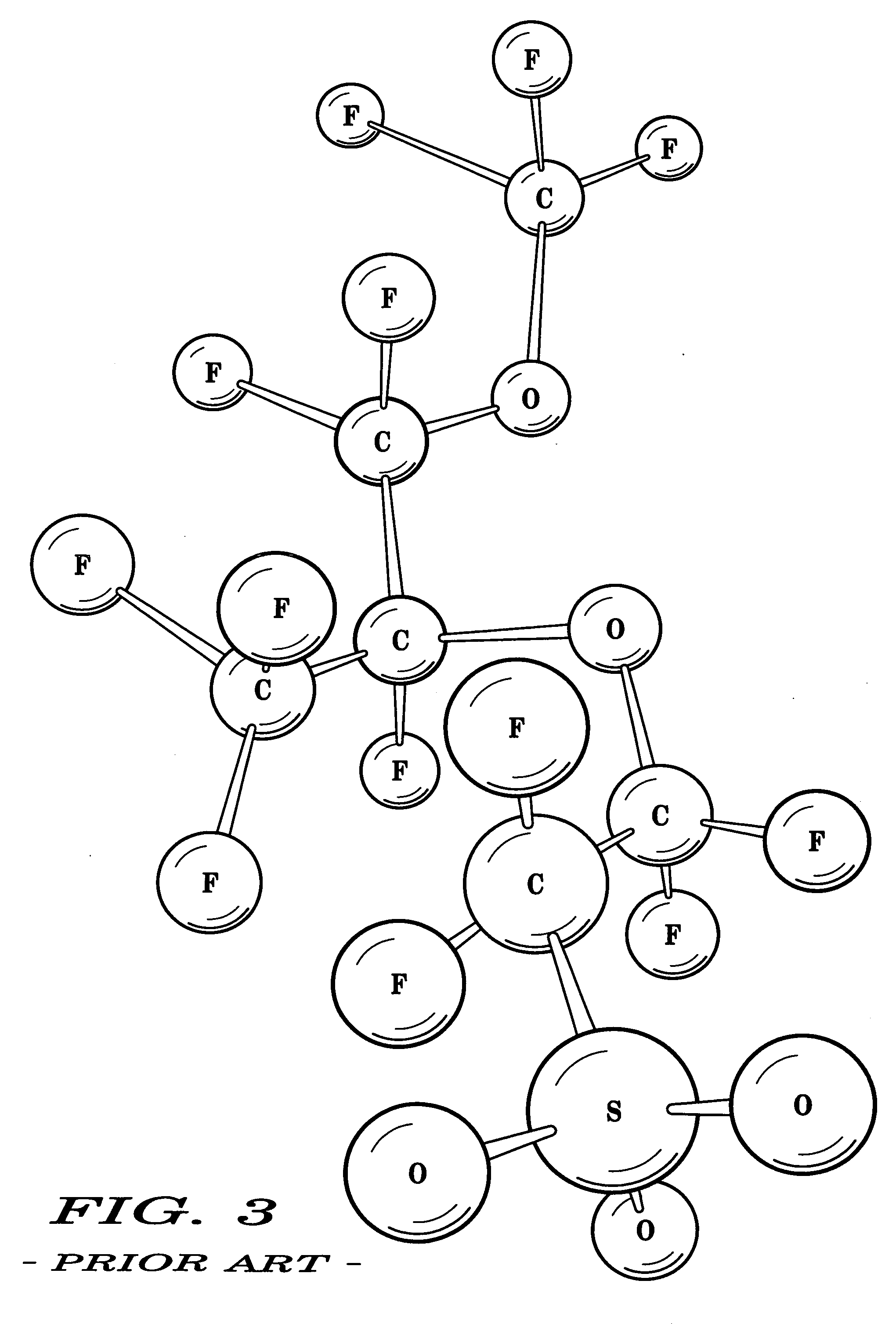
Hydrophilic side-chain polymer, electrolyte membranes
a side chain polymer and electrolyte technology, applied in the direction of non-aqueous electrolyte cells, cell components, electrochemical generators, etc., can solve the problems of reducing the efficiency of the fuel cell, depleting the chemical components of the electrode, and not having a molecular level understanding of electro-osmosis, so as to prevent or improve the increase of electro-osmotic drag and increase swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The following descriptions are of exemplary embodiments of the invention and the inventor's conception of the best mode and are not intended to limit the scope, applicability or configuration of the invention in any way. Rather, the following description is intended to provide convenient illustrations for implementing various embodiments of the invention. As will become apparent, changes may be made in the function and / or arrangement of any of the elements described in the disclosed exemplary embodiments without departing from the spirit and scope of the invention.
[0032] Various representative implementations of the present invention may be applied to any composition of matter, system and / or method for the characterization of proton dissociation and transport as they may relate to hydrophilic components of hydrated ionomeric materials. A detailed description of an exemplary application, namely a composition of matter and a method for altering the differential hydrophilicity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com