Test device and method for colored particle immunoassay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
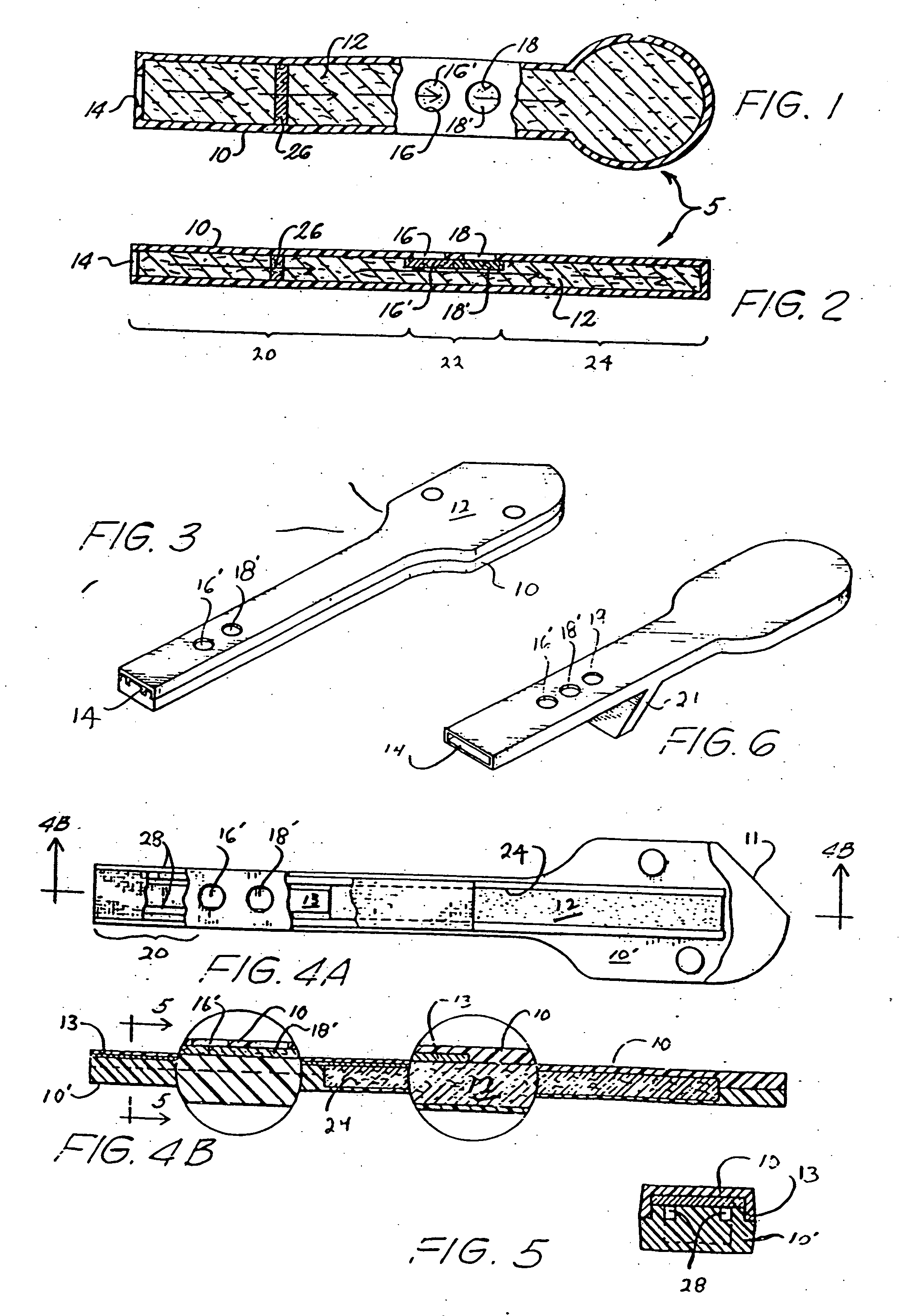
[0044] The currently preferred test device embodying the invention is shown in FIGS. 3, 4A, 4B, and 5. A modification of the device depicted in FIG. 3 is shown in FIG. 6, and includes a second control site 19 in addition to control site 16′ and test site 18′, as well as a stand 21 useful for maintaining the test cell in an incline position with the reservoir downhill. When a test sample is applied to inlet 14, gravity as well as sorption aids in transporting the sample along the flow path.
[0045] As shown in FIGS. 3, 4A, 4B, and 5, the preferred test cell of the invention differs from the exemplary device discussed above and shown in FIGS. 1 and 2 in certain of its more specific internal features. Specifically, the casing comprises a pair of interfitting polymeric parts including a U-shaped top part 10 which, when the device is assembled, interfits with lower part 10′. Top and bottom parts 10 and 10′ may be connected through a hinge region 11. The bottom section 10′ defines a pair o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com