Macrophage derived chemokine (MDC), MDC analogs, MDC inhibitor substances and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of a cDNA Encoding MDC
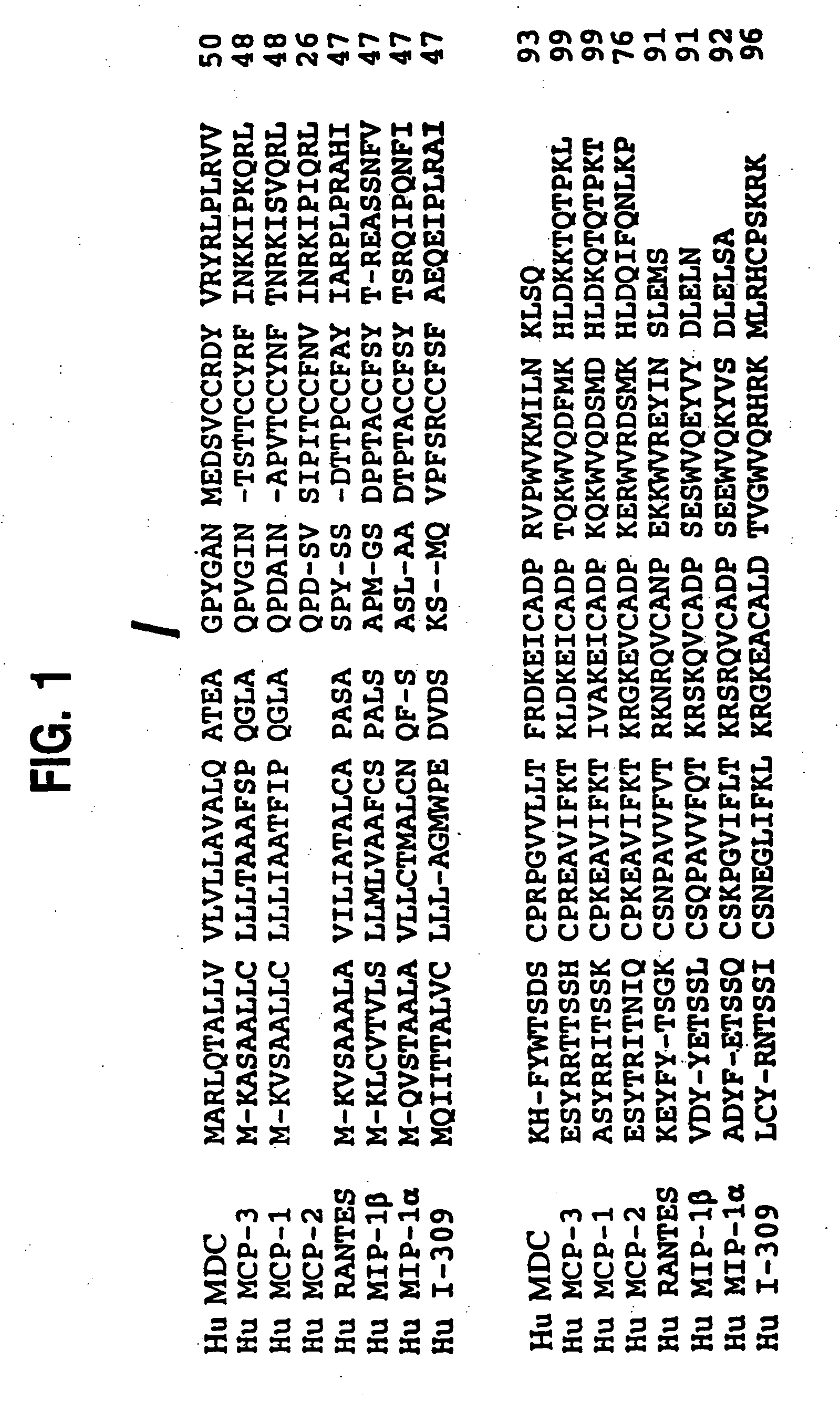
[0119] A partial cDNA for a new C-C chemokine, designated pMP390, was isolated from a macrophage cDNA library as described in U.S. patent application Ser. No. 08 / 939,107, filed Sep. 26, 1997, and in related international publication number WO 96 / 40923, both of which are incorporated herein by reference. Sequence comparisons were performed on Dec. 14, 1994, by the BLAST Network Service of the National Center for Biotechnology Information (e-mail: “blast@ncbi.nlm.nih.gov”), using the alignment algorithm of Altschul et al., J. Mol. Biol., 215: 403-410 (1990). The sequence analysis revealed that a portion of the isolated macrophage cDNA clone designated pMP390 contained a gene sequence having approximately 60-70% identity with previously-identified chemokine genes, including the human MCP-3 gene and rat MIP-1β gene.
[0120] The 2.85 kb cDNA insert of pMP390 was subcloned into the vector pBluescript SK− (Stratagene, La Jolla Calif.) to facilitate complete ...
example 2
Isolation of Additional cDNA Clones Having the Complete MDC Coding Sequence
[0121] Using the pMP390 cDNA clone isolated in Example 1, additional cDNA clones were isolated from the same human macrophage cDNA library, these additional cDNAs containing additional 5′ sequence and encoding the complete amino acid sequence of a macrophage derived chemokine. The additional cloning and sequencing is described in detail in U.S. Ser. No. 08 / 939,107 and WO 96 / 40923, incorporated herein by reference.
[0122] Of the additional clones, clones designated pNT390-12 and pMP390B contained the largest additional 5′ coding sequence, each extending an additional 72 nucleotides upstream of the sequence previously obtained from the cDNA clone pMP390. A composite DNA sequence, herein designated MDC cDNA, was generated by alignment of the pMP390 and pMP390-12 cDNA sequences. This 2923 base pair composite cDNA sequence, and the deduced amino acid sequence of the chemokine MDC, are set forth in SEQ ID NOs: 1 a...
example 3
Determination of MDC Gene Expression in Human Tissues
[0125] Northern blot analysis were conducted to determine the tissues in which the MDC gene is expressed.
[0126] A radiolabeled pMP390 5′ fragment which corresponds to the region of the MDC cDNA encoding the putative mature form of MDC plus 163 bases of the adjacent 3′ noncoding region was used to probe Multiple Tissue Northern blots (Clontech, Palo Alto, Calif.) containing RNA from various normal human tissues. The probe was denatured by boiling prior to use, and the hybridizations were conducted according to the manufacturer's specifications. Autoradiographs were exposed 5 days at −80° C. with 2 intensifying screens.
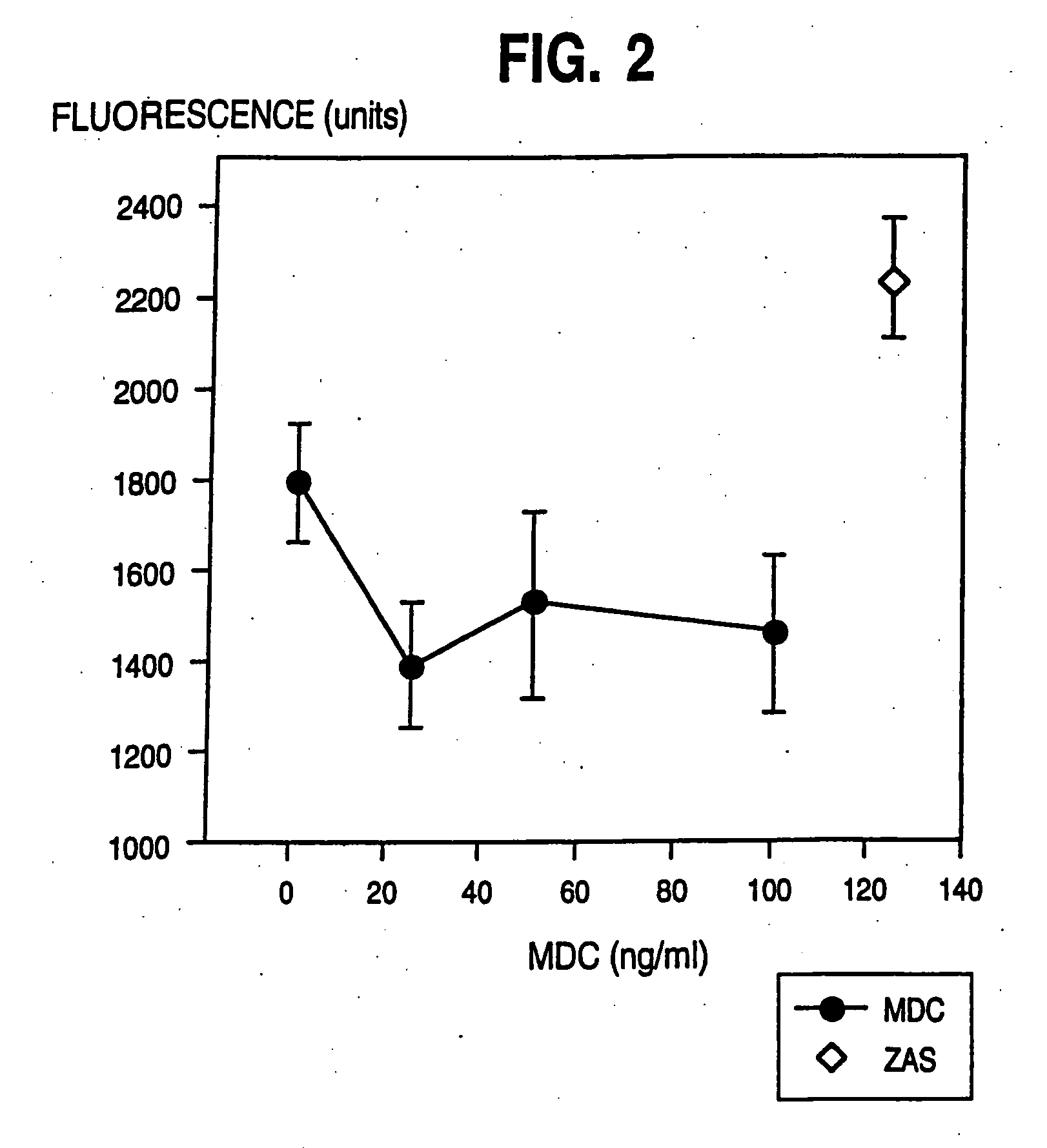
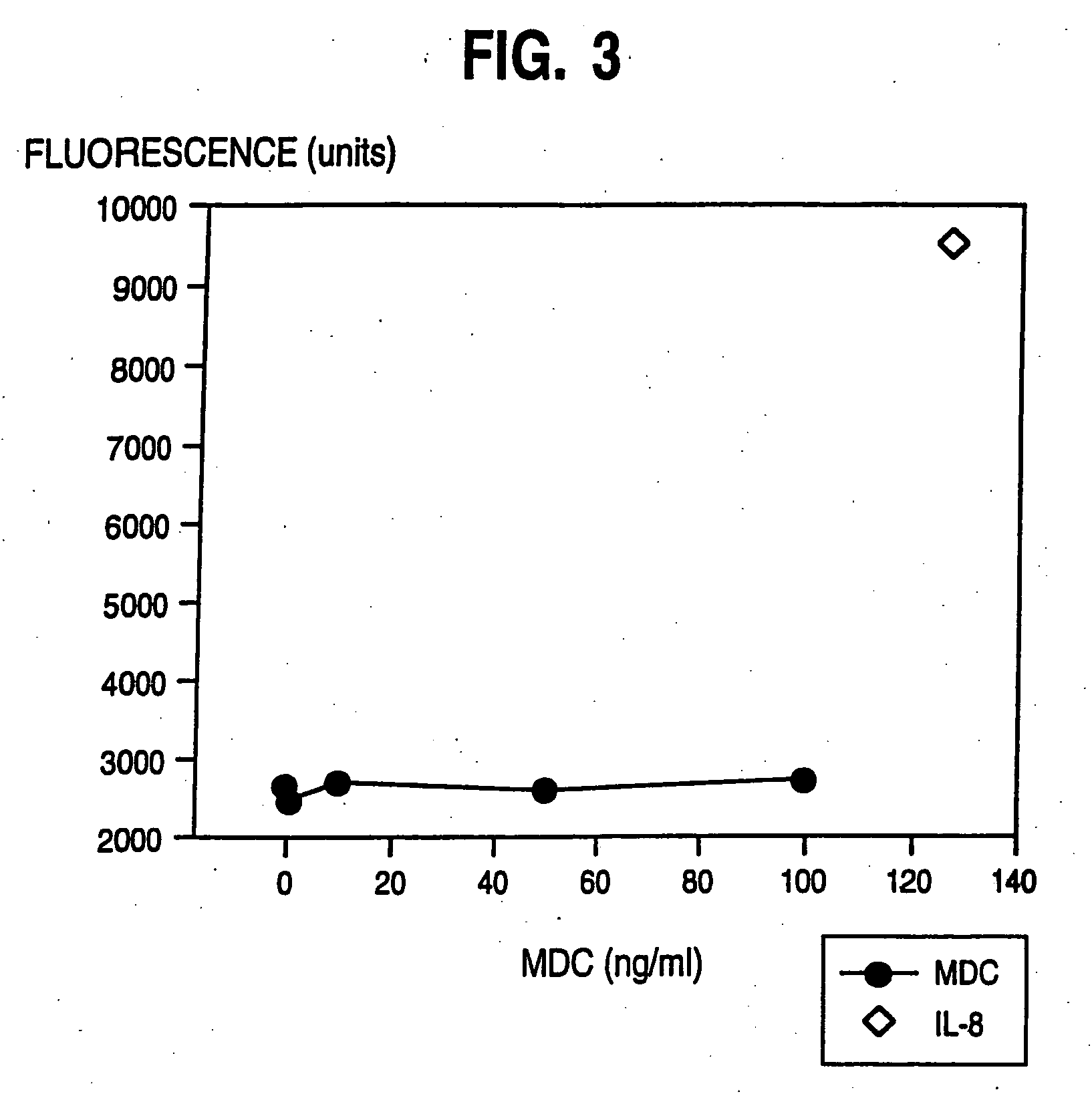
[0127] The greatest MDC gene expression was observed in the thymus, with much weaker expression detectable in spleen and lung tissues. Expression of MDC in tissue from the small intestine was at even lower levels, and no expression was detected in brain, colon, heart, kidney, liver, ovary, pancreas, placenta, prost...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com