Imageable biopsy site marker
a biopsy site and marker technology, applied in the field of markers, can solve the problems of significantly reducing the mortality rate caused by this form of cancer, days or even a week before, and achieve the effects of accurate visualization of the boundary, easy or enhanced visualization of the biopsy cavity boundary, and accurate excise and remove a quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] The following specification taken in conjunction with the drawings sets forth the preferred embodiments of the present invention. The embodiments of the invention disclosed herein are the best modes contemplated by the inventors for carrying out their invention in a commercial environment, although it should be understood that various modifications can be accomplished within the parameters of the present invention.
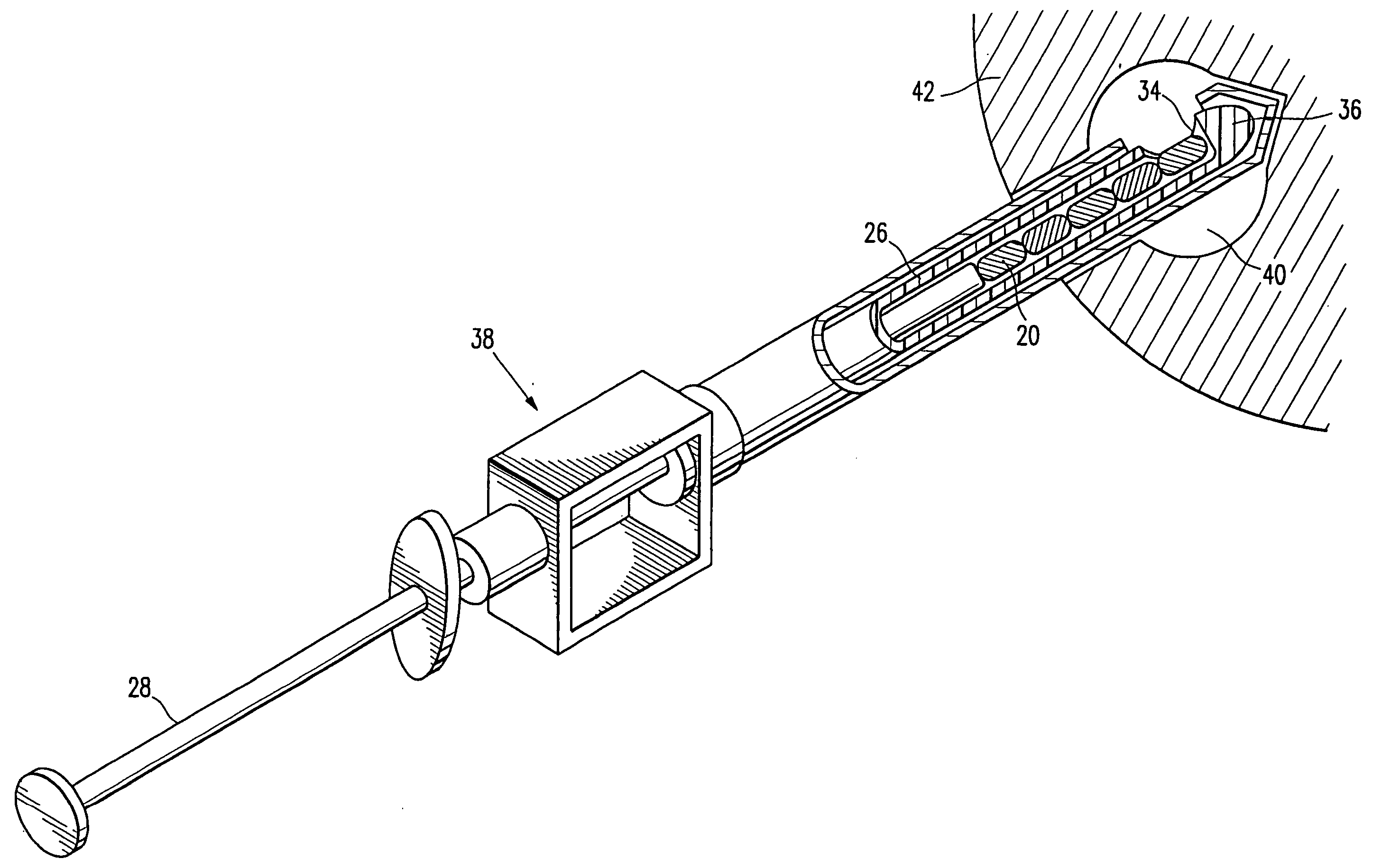
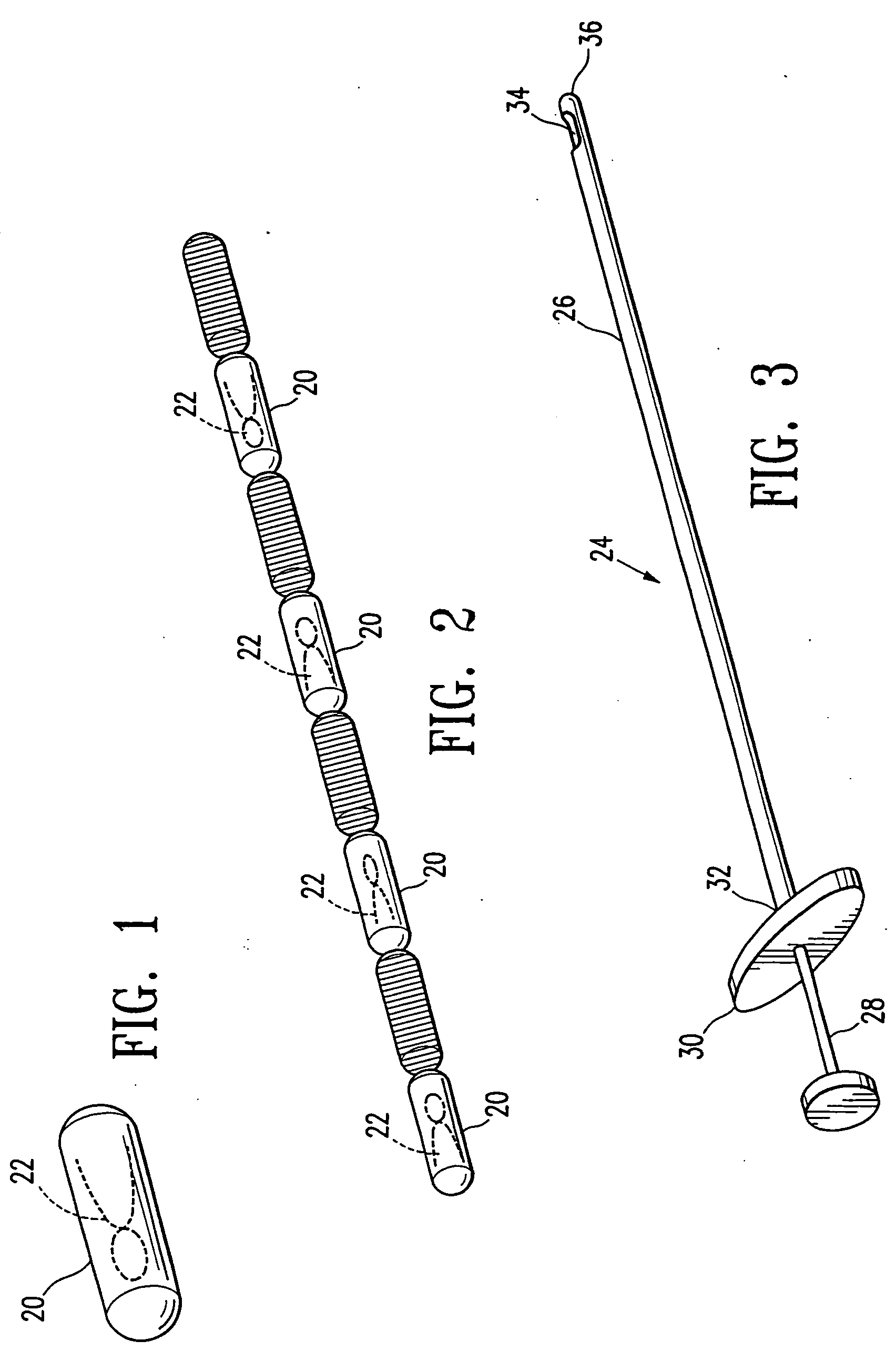
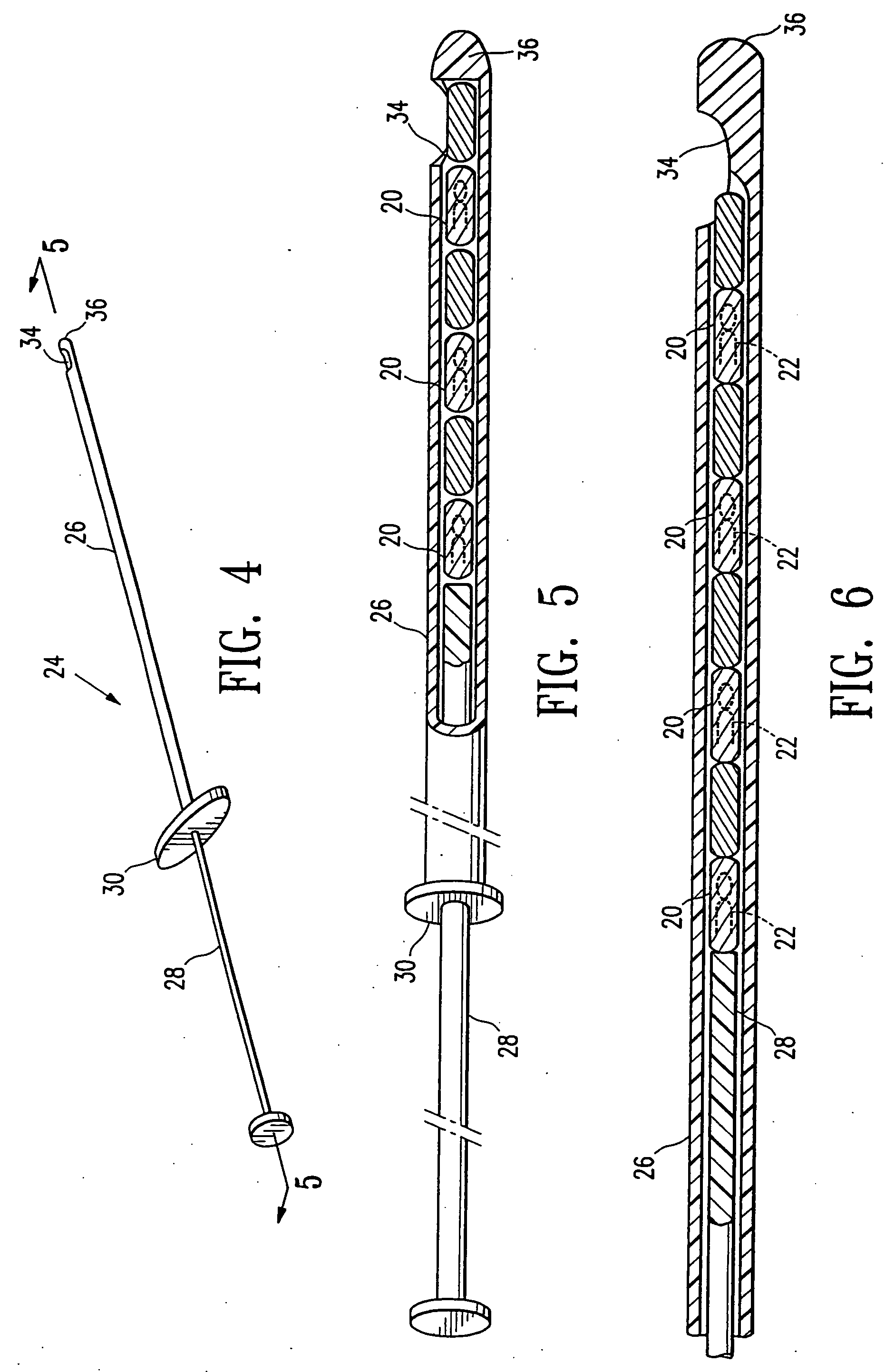
[0058] Referring now to the drawing figures and particularly to FIGS. 1 and 2, a body 20 of gelatin or reconstituted collagen in the shape of a pellet that includes or incorporates a radio-opaque marker 22 of a definite shape is disclosed. The gelatin or reconstituted collagen body 20 can be of virtually any shape or configuration, however the herein shown shape of a cylinder or pellet is preferred. The gelatin body of pellet 20 is of such size that several of the pellets can be deposited in a biopsy site, such as a typical biopsy site obtained by using the vacuum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com