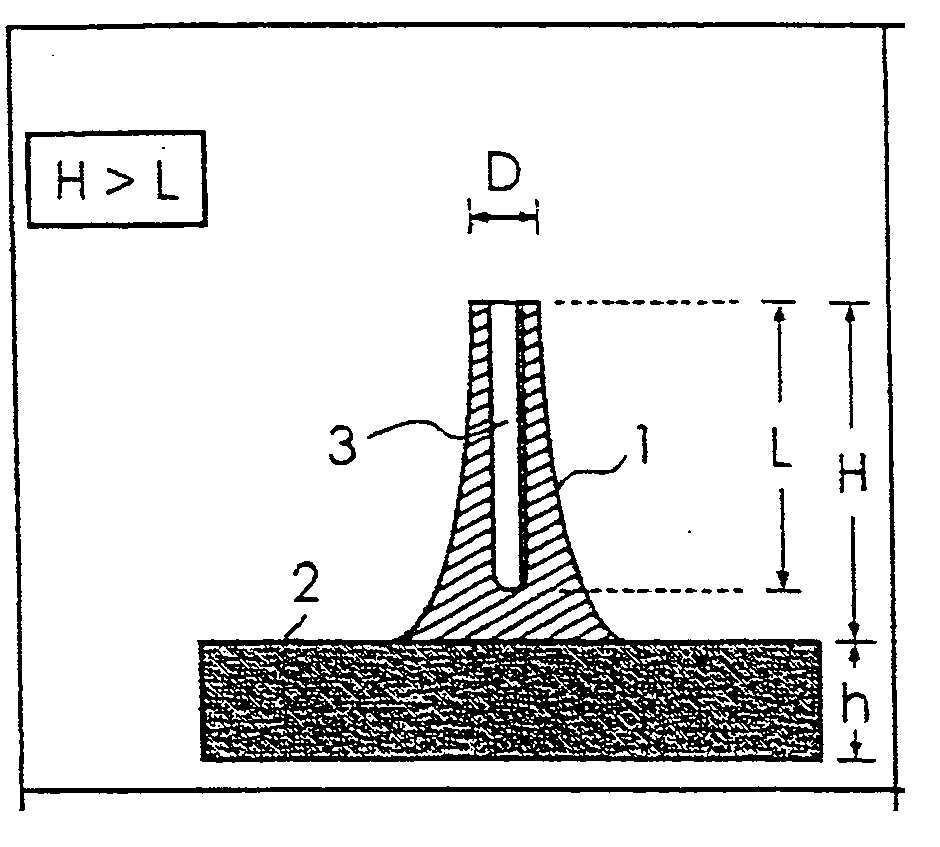
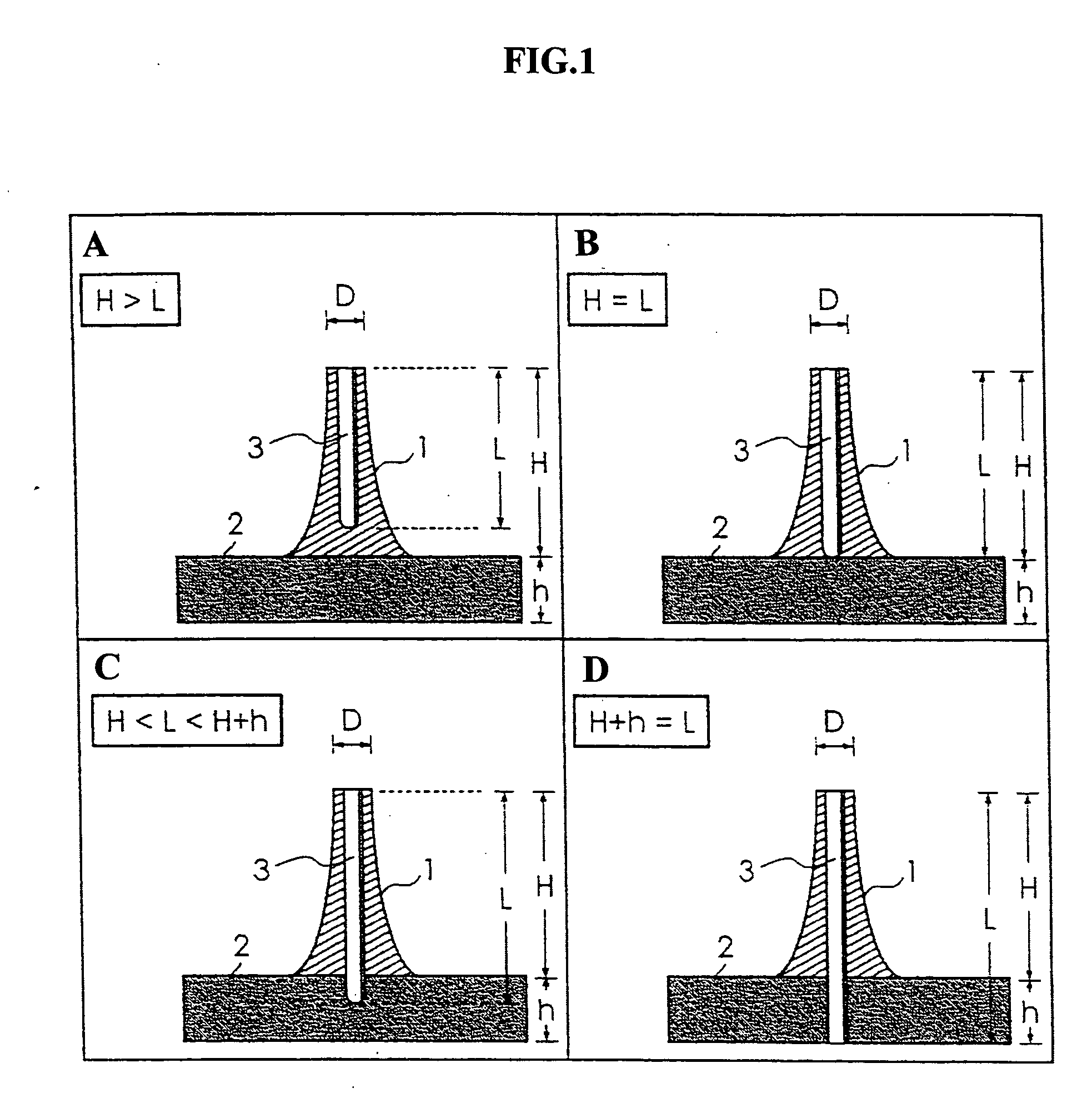
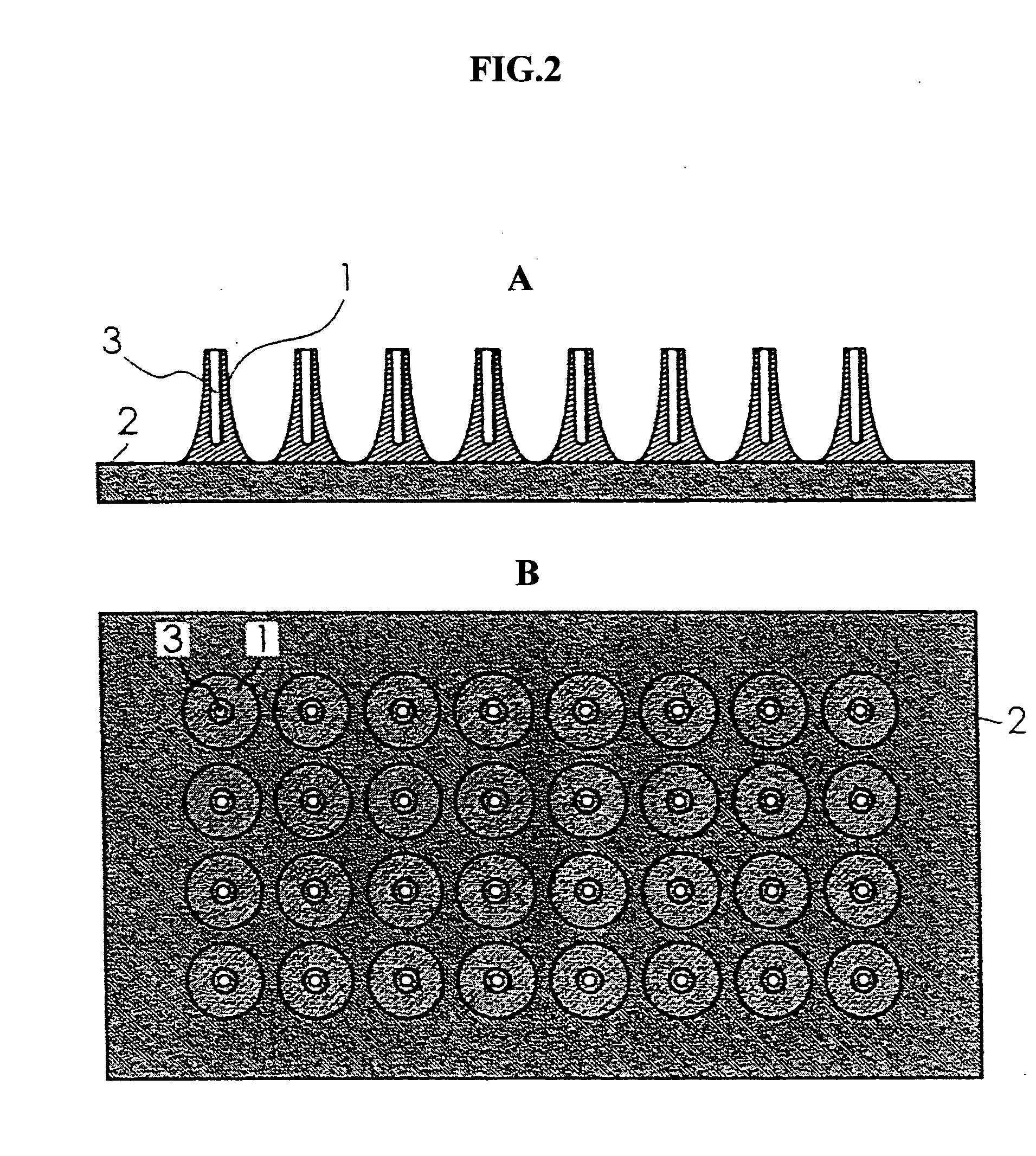
Pad base for transdermal administration and needle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0043] The pad bases for endermism of Examples of the present invention are illustrated below together with examples of the specific production process.
examples 1 to 3
[0044] As a section bar for molding the minute needles, a section bar in which stainless steel wires (thin metal wires) having a length of about 30 mm and a diameter φ of 280 μm were vertically inserted by 5 wires in longitudinal and by 6 wires in a reticular pattern at an interval of 2 mm in a rubber plate was prepared. Then, the edges of stainless steel wires of the above-mentioned section bar were perpendicularly brought in contact with the bottom of a stainless steel dish and, 3 ml of a chloroform solution containing polylactic acid with a molecular weight of 101700 was poured in the stainless steel dish. After that, these were left alone, chloroform was evaporated by naturally drying and the polylactic acid was solidified. Then, the stainless steel wires were taken out from the stainless steel dish to obtain a pad base for endermism. Further, solutions with 5, 6 and 7% by weight as the concentration of polylactic acid in the above-mentioned chloroform solution containing polyla...
examples 4 to 6
[0046] A similar section bar of the minute needles as the above-mentioned Examples 1 to 3 was used and the edges of stainless steel wires of the above-mentioned section bar were perpendicularly brought in contact with the bottom of a stainless steel dish. 3 ml of a chloroform solution containing polylactic acid with a molecular weight of 67400 was poured in the stainless steel dish, left alone, and the polylactic acid was solidified by natural drying. Then, the stainless steel wires were taken out from the stainless steel dish to obtain a pad base for endermism. Further, solutions with 10, 11 and 12% by weight as the concentration of polylactic acid in the above-mentioned chloroform solution containing polylactic acid were prepared, and pad bases which were obtained for the respective solutions were referred to as Examples 4, 5 and 6.
[0047] Any of the above-mentioned Examples 4 to 6 was a pad base for endermism which had a plural number of the minute needles with a shape as shown i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com