Olefinic metathesis in the presence of phenolic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Phenol Enhancement Effect
[0066] The general experimental procedure was followed without addition of any phenol and with the addition of 500 eq of phenol.
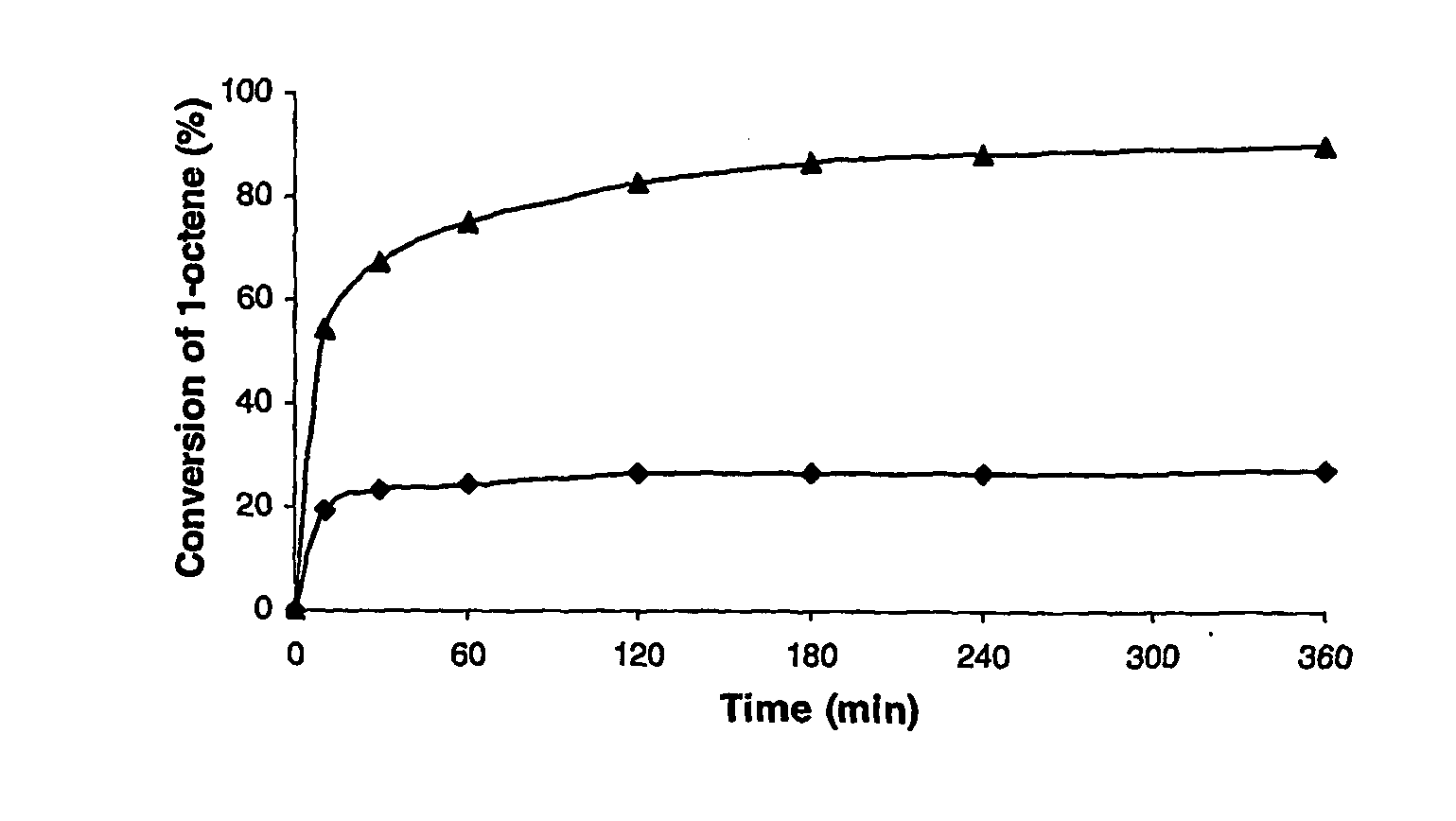
[0067] An increased conversion of 1-octene to the desired 7-tetradecene product was observed for the metathesis of 1-octene when 500 equivalents of phenol was added to the reaction mixture (Table 1) compared to the reaction where no phenol was added. Under these conditions, no detectable (by GC) amounts of isomerised octene or secondary metathesis products (SMP's) were observed. Furthermore, the catalyst was active even after four hours, in marked contrast to the control experiment where no phenol was added. In an effort to verify the results obtained above, the reaction was repeated. These results indicate that the reaction can be readily reproduced.
TABLE 1Metathesis of 1-octene with G1 (no phenol) vs G1 + phenol (500 eq) at50° C.Molar % Conversion of 1-OcteneConditionsAfter 3 hG1 with no phenol added26.5%G1 + 500 eq phenol...
example 2
Effect of Phenol Concentration
[0069] The 1-octene metathesis reactions were performed following the general experimental procedure and using 200, 500 and 1000 equivalents of phenol, respectively. The results are shown in Table 2. Under these conditions, optimum performance is achievable with either 500 or 1000 equivalents of phenol.
TABLE 2Metathesis of 1-octene with G1 + phenol (200, 500 and 1000eq).Molar % Conversion of 1-OcteneConditionsAfter 3 hG1 with no phenol added26%G1 + 200 eq phenol63%G1 + 500 eq phenol82%G1 + 1000 eq phenol77%
example 3
Effect of Substitution on Phenol
[0070] In an effort to asses the effect of substitution on the benzene ring of the phenol on the formation of 7-tetradecene in 1-octene metathesis, 500 equivalents of compounds 1-6 were added to the reaction mixture, following the general experimental procedure. The results are summarized in Table 3.
TABLE 3Metathesis of 1-octene with substituted phenolsMolar % Conversion of 1-ConditionsOctene After 3 hG1 with no phenol added26.5%G1 + 500 eq 4-Cl-phenol (Compound 3)58.2%G1 + 500 eq 4-CF3-phenol (Compound 4)64.8%G1 + 500 eq 4-I-phenol (Compound 5)71.0%G1 + 500 eq 4-OMe-phenol (Compound 1)71.8%G1 + 500 eq phenol82.0%G1 + 500 eq 4-F-phenol (Compound 6)86.0%G1 + 500 eq 4-Me-phenol (cresol)91.1%(Compound 2)
[0071] These results show that the addition of 500 equivalents of p-cresol (compound 2) afforded very similar (but slightly better) yields compared to those obtained with phenol. 4-Methoxyphenol (compound 1) gave slightly lower conversions than phenol ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com