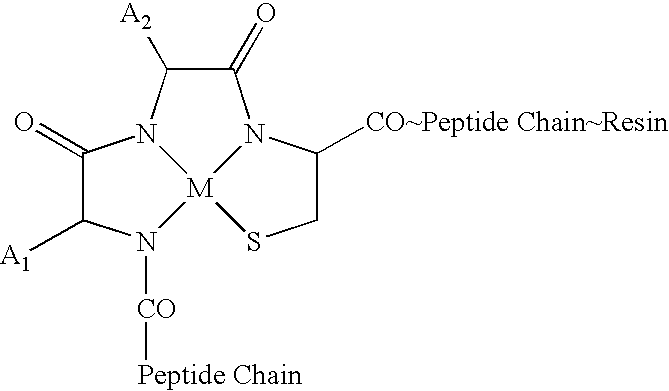
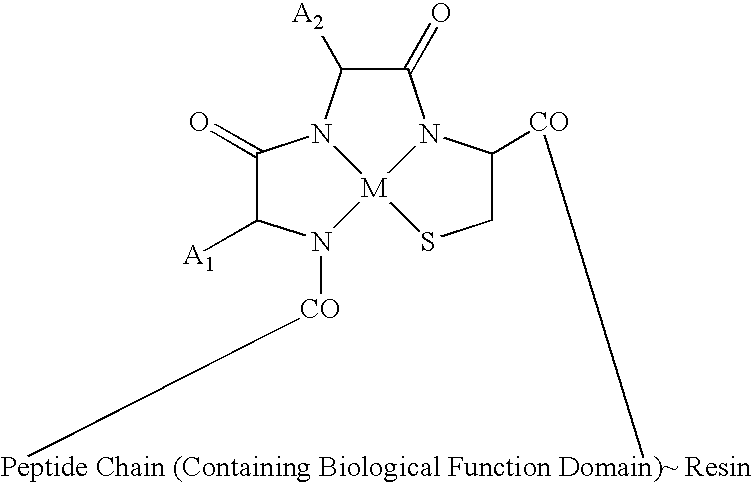
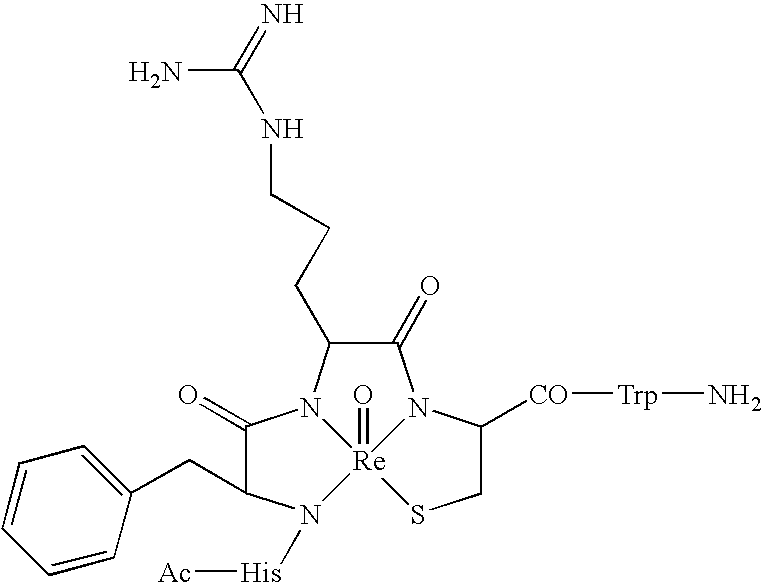
Melanocortin Metallopeptode Combinatorial Libraries and Applications
a metallopeptode and melanocortin technology, applied in the field of metallopeptode combinatorial libraries and applications, can solve the problems of soluble library approach applicability to high affinity systems, totally misleading mice, and inability to tolerate soluble libraries, etc., to achieve rapid and efficient complexation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Development of a Prototype Metallopeptide Library for the Melanocortin Receptor
[0190] The library design was based on the tetrapeptide message sequence, His-Phe-Arg-Trp (6-9 sequence) (SEQ ID NO:1), of α-MSH. This sequence exists as a reverse turn, making it suitable for conversion into a metallopeptide format of this invention. In this approach metallopeptides were designed around a tripeptide N3S1 MBD designed for a rhenium metal ion. The MBD was derivatized to yield the pentapeptide Ac-His-Phe-Arg-Cys-Trp-NH2 (SEQ ID NO:8) as a putative candidate for melanocortin (“MC”) receptors. Further refinements in the structure were made in response to other considerations, including the chirality of amino acid side chains, yielding a template structure Ac-His-D-Phe-Arg-Cys-Trp-NH2. The structure of this peptide after binding to rhenium is:
[0191] The template structure was used to define a small combinatorial library utilizing split synthesis methodologies. The final template selected fo...
example 2
Design and Synthesis of Melanocortin Receptor-Specific Metallopeptide Library
[0193] The library was rationally designed based upon data relating to melanocortin receptors and peptide sequences specific to the melanocortin receptors, including melanotropin side-chain pharmacophores, D-Phe7 and Trp9, that interact with a hydrophobic network of receptor aromatic residues in transmembrane regions 4, 5, 6, and 7. Based on this design criterion, a pharmacophore for the melanocortin receptor was preliminarily defined, and a combinatorial library designed for identification of potent and receptor-selective agonists.
[0194] Based on the design criteria, the putative structure R-Aaa-Baa-L-Cys-Caa-NH2 was selected, in which each of Aaa, Baa and Caa are selected from L- or D-isomers of 2-Nal (1), Phe (2), Trp (3), Tyr (4) and Ala (5), so that any one of the foregoing can be substituted for any one of Aaa, Baa or Caa. In the nomenclature adopted for the library design, the five amino acids were...
example 3
Screening of Melanocortin Receptor-Specific Library
[0202] Metallopeptide library pools are screened for MC4-R receptor and MC1-R receptor binding activity in high throughput screening assays. The MC receptor-binding assay uses membrane preparations from B16-F1 or B16-F10 melanoma cells as the source of MC receptor. Cell membranes prepared from MC4-R-expressing 293 cells and negative control, untransfected 293 cells, are substituted for B16-F1 or B16-F10 melanoma cell membranes in MC4-R specific binding assays. The MC receptor-binding assays use the Millipore Multi-Screen System and are performed in 96-well Millipore filter plates (Durapore, 0.45 mm porosity) pre-blocked with 0.5% bovine serum albumin in phosphate buffered saline. Cell membrane preparations (12.5 μg / well) are incubated with 0.4 nM 125I-NDP-MSH in HEPES Buffer containing 0.2% bovine serum albumin. Non-specific binding is determined by addition of 10−6 M α-MSH or 10−7 M NDP-MSH. Metallopeptides to be tested are added ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com