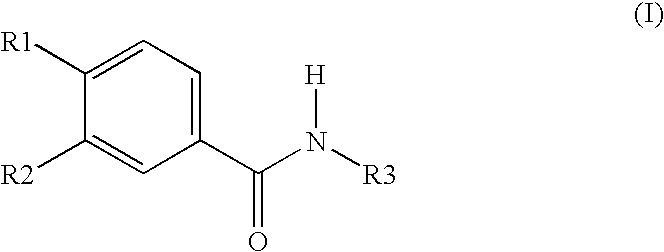
Oral dosage form containing a PDE 4 inhibitor as an active ingredient and polyvinylpyrrolidone as excipient
a technology of pde 4 inhibitor and oral dosage form, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of difficult to produce suitable dosage forms, difficult to control or sustain the release of slightly soluble active ingredients, and complicated production of dosage forms with controlled or sustained release of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Production of Tablets of the Invention
example a
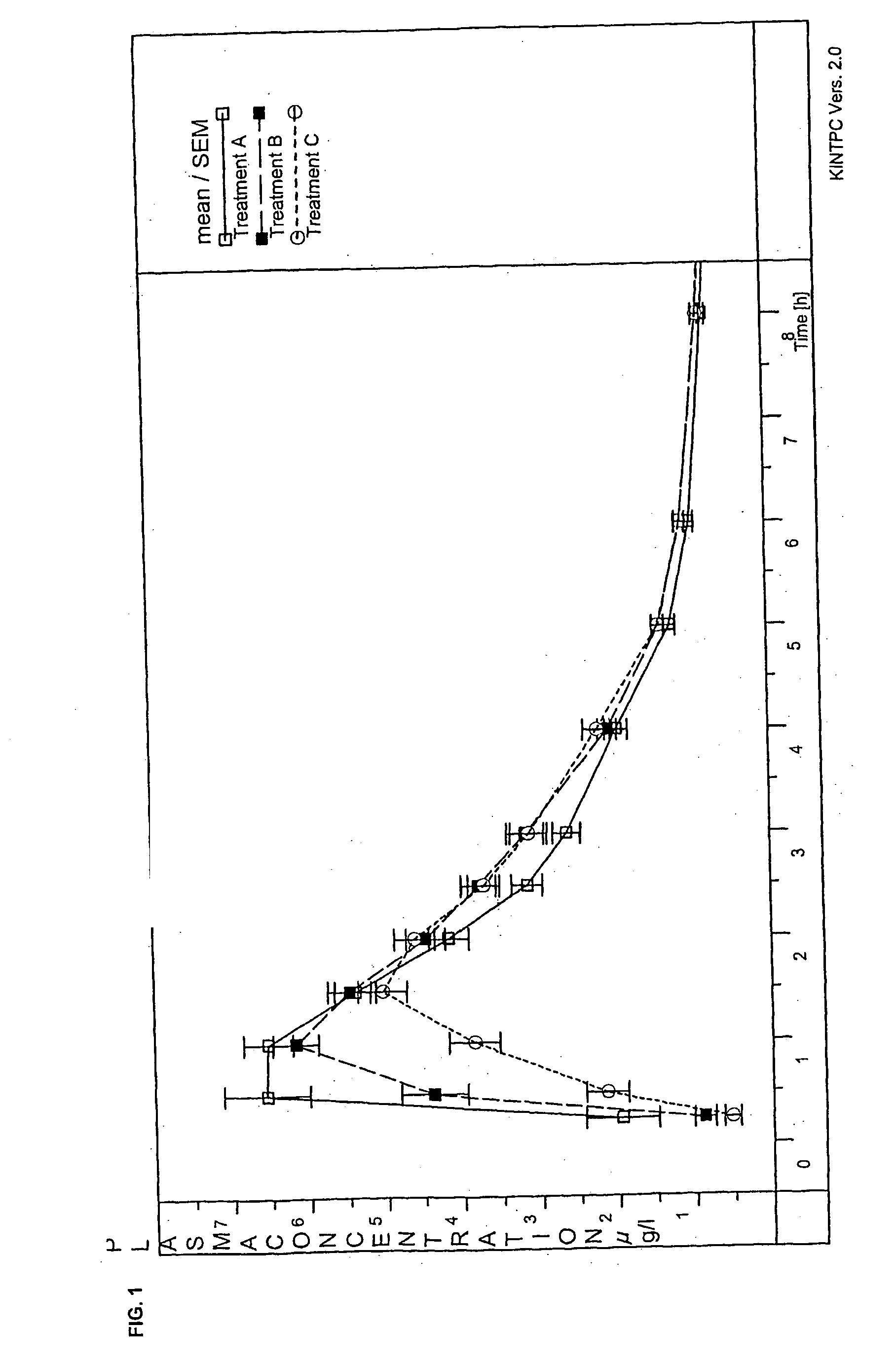
[0106] Weight Based on a Tablet Containing 0.1 mg of Roflumilast
1.Roflumilast (micronized)0.100mg2.Lactose monohydrate49.660mg3.Corn starch13.390mg4.Polyvidone K901.300mg5.Magnesium stearate (vegetable)0.650mgTotal65.100mg
[0107] Production: (1) is mixed with part of (3), and a trituration is produced in a planetary mill. The trituration is put together with (2) and the remaining amount of (3) in the product container of a fluidized bed granulation system, and a 5% granulation solution of (4) in purified water is sprayed on and dried under suitable conditions. (5) is added to the granules, and the mixture obtained after mixing is compressed in a tablet press to tablets having an average weight of 65.1 mg.
example b
[0108] Weight Based on a Tablet Containing 0.125 mg of Roflumilast
1.Roflumilast0.125mg2.Lactose monohydrate49.660mg3.Corn starch13.390mg4.Polyvidone K901.300mg5.Magnesium stearate (vegetable)0.650mgTotal65.125mg
[0109] Production: (1) is mixed with part of (3), and a trituration is produced in a planetary mill. The trituration is put together with (2) and the remaining amount of (3) in the product container of a fluidized bed granulation system, and a 5% granulation solution of (4) in purified water is sprayed on and dried under suitable conditions. (5) is added to the granules, and the mixture obtained after mixing is compressed in a tablet press to tablets having an average weight of 65.125 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com