Therapeutic combination
a combination and therapy technology, applied in the field of therapeutic combination, can solve the problems of adverse cardiac events, hypertension intervention trials that have failed to demonstrate full normalization of cardiovascular mortality, and patients who undergo ptca or other surgical procedures designed to treat angina pectoris frequently experience complications, so as to slow down the progression of atherosclerotic plaques
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
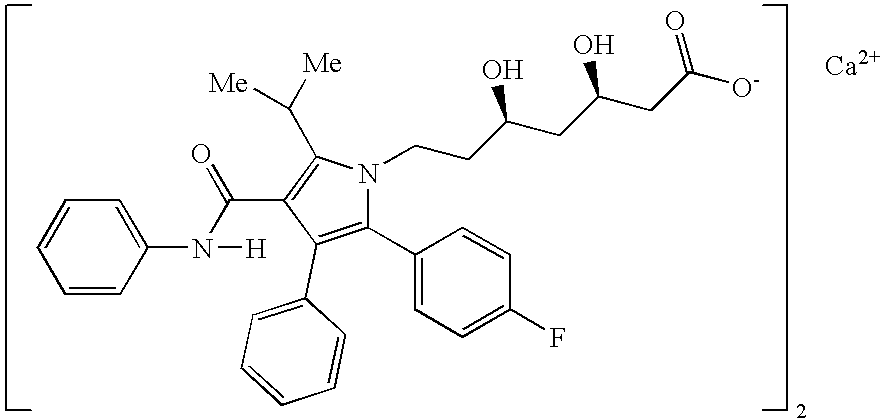
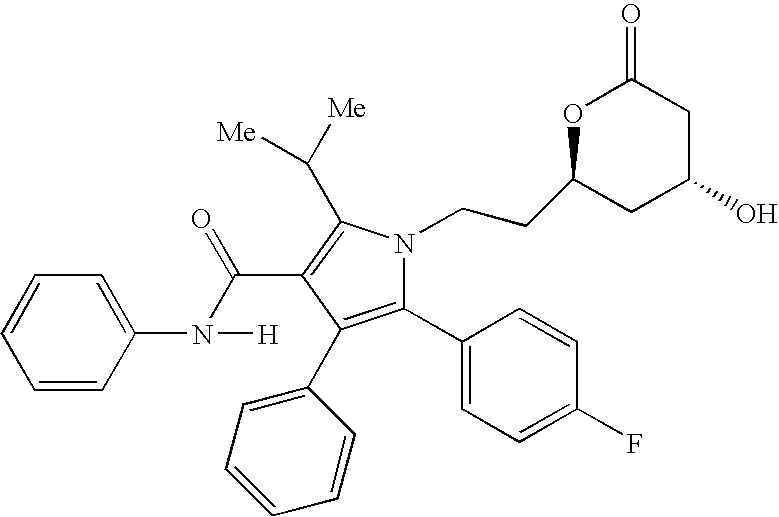
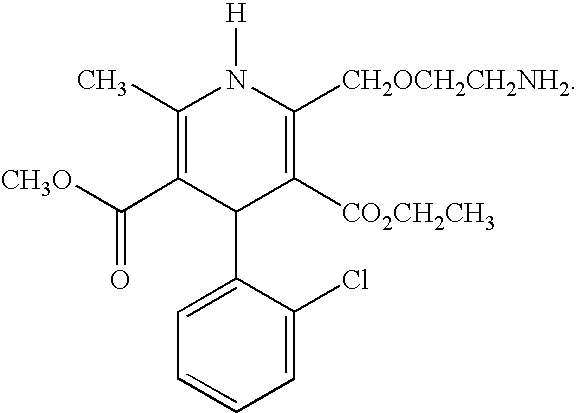
[0142] The pharmaceutical compositions of this invention comprise amlodipine or a pharmaceutically acceptable acid addition salt thereof and / or atorvastatin or a pharmaceutically acceptable salt thereof.
[0143] Amlodipine may readily be prepared as described in U.S. Pat. No. 4,572,909 which is incorporated herein by reference. Amlodipine besylate, which is currency sold as Norvasc®, may be prepared as described in U.S. Pat. No. 4,879,303, which is incorporated herein by reference. Amlodipine and amlodipine besylate are potent and long lasting calcium channel blockers.
[0144] The expression “pharmaceutically-acceptable acid addition salts” is intended to define but is not limited to such salts as the hydrochloride, hydrobromide, sulfate, hydrogen sulfate, phosphate, hydrogen phosphate, dihydrogenphosphate, acetate, besylate, succinate, citrate, methanesulfonate (mesylate) and p-toluenesulfonate (tosylate) salts.
[0145] Other acid addition salts of amlodipine may be prepared by reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time lipid levels | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com