Gene differentially expressed in breast and bladder cancer, and encoded polypeptides
a human gene and gene technology, applied in the field of human genes, can solve the problems of unfavorable broad-effect tumor vaccine development, no a priori reason why random mutation and systematic gene deregulation cannot both give rise to new immunogenic expression, and technological challeng
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Differential Expression of C35 in Human Breast Carcinoma
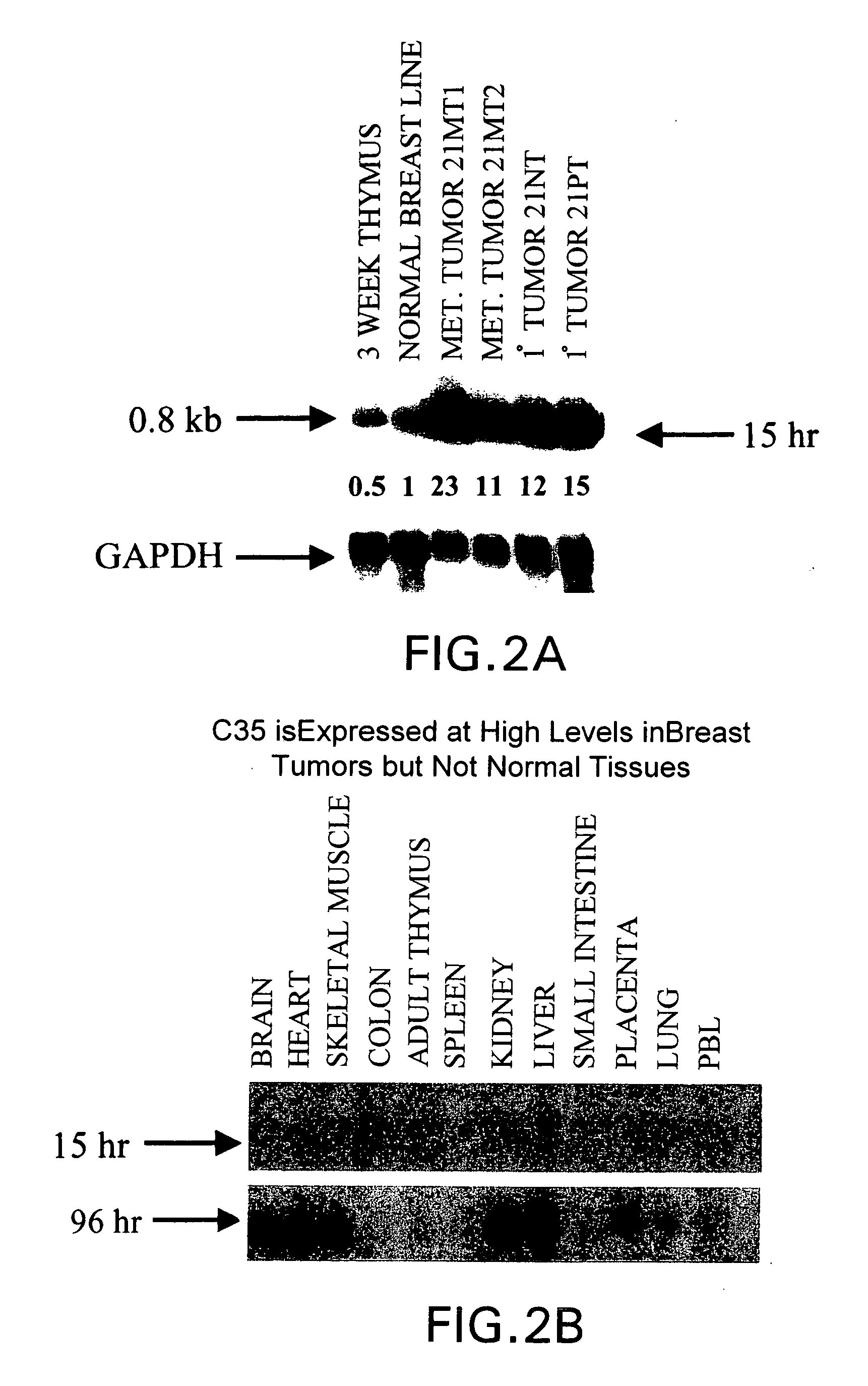
[0259] The present inventors have characterized a full-length cDNA representing a gene, C35, that is differentially expressed in human breast and bladder cancer (FIG. 1). A 348 base pair DNA fragment of C35 was initially isolated by subtractive hybridization of poly-A RNA from tumor and normal mammary epithelial cell lines derived from the same patient with primary infiltrating intraductal mammary carcinoma. (Band, V. et al., Cancer Res. 50:7351-7357 (1990). Employing primers based on this sequence and that of an overlapping EST sequence (Accession No. W57569), a cDNA that includes the full-length C35 coding sequence was then amplified and cloned from the SKBR3 breast tumor cell line (ATCC, HTB-19). This C35 cDNA includes, in addition to the 348 bp coding sequence, 167 bp of 3′ untranslated region.
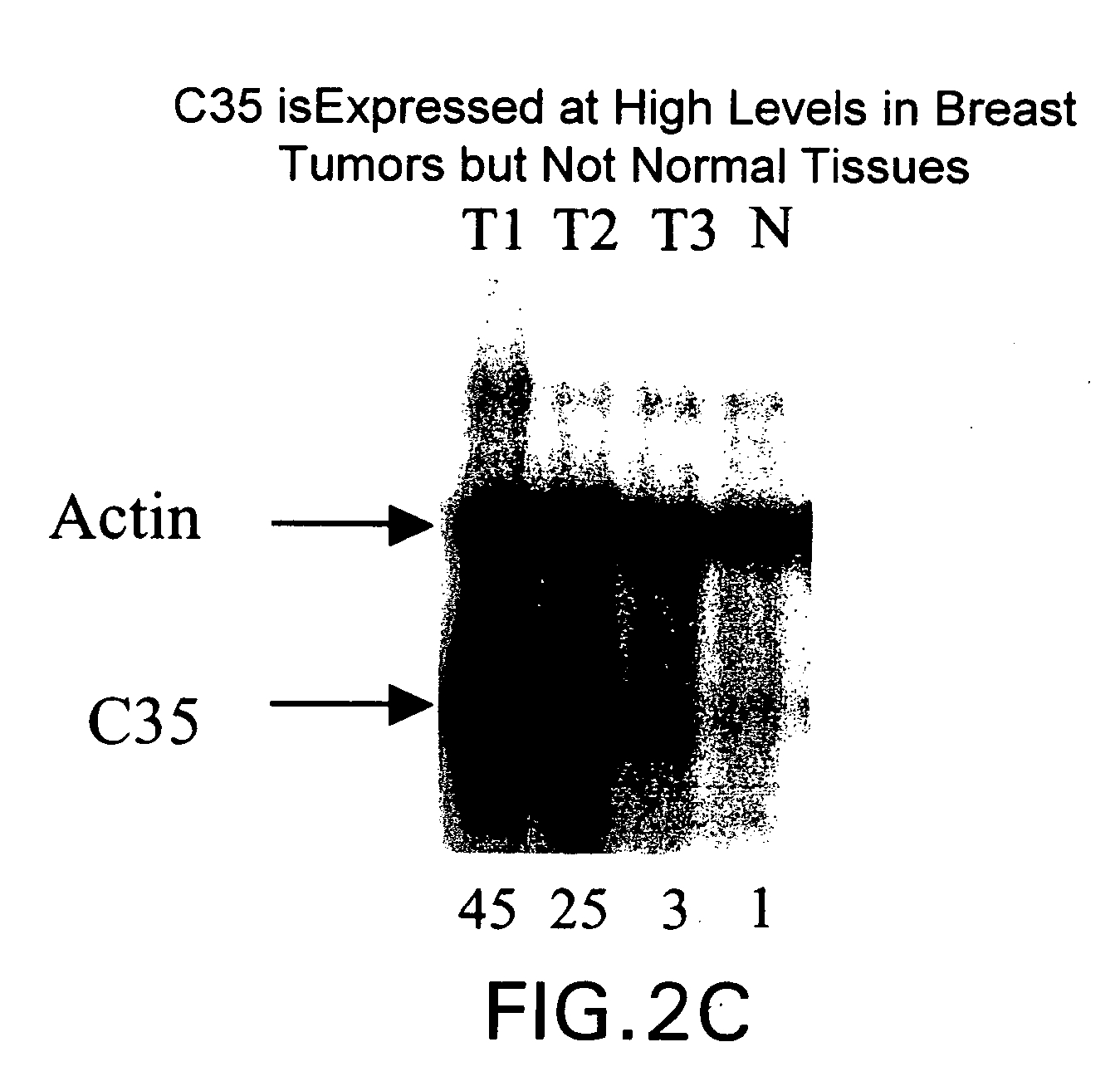
[0260] Differential expression of the C35 sequence is demonstrated in FIG. 2 panel A which compares expression levels of clone C35 ...
example 2
C35 Specific CTL are Cytolytic for C35 Positive Breast Tumor Cells
[0262] Although a gene product may be overexpressed in tumor cells, as is the case for C35, it is immunologically relevant only if peptides derived from that gene product can be processed and presented in association with MHC molecules of the tumor cells. It is conceivable that for any given gene product either no peptides are produced during the cellular degradation process that satisfy the requirements for binding to the MHC molecules expressed by that tumor, or, even if such peptides are generated, that defects in transport or competition for MHC molecules by other tumor peptides would preclude presentation of any peptides from that specific gene product. Even if relevant tumor peptides are processed and presented in association with human MHC in the tumor cells, it must in all cases be determined whether human T cells reactive to these peptides are well-represented in the repertoire or whether T cells may have be...
example 3
C35 Expression on the Membrane of Breast Carcinoma Cells
[0265] To determine whether the C35 polypeptide product is expressed on the surface of tumor cells, a C35 specific antiserum was prepared. BALB / c mice were immunized with syngeneic Line 1 mouse tumor cells that had been transduced with retrovirus encoding human C35. Mice were bled following a series of two or more immunizations. The immune sera were employed to detect surface expression of C35 protein by flow cytometry on three breast tumor cell lines representing high (21NT), intermediate (SKBR3), and low (MDA-MB-231 levels of expressionof the C35 transcript in Northern blots (see FIG. 4). 1×105 breast tumor cells were stained with 3.5 microliters of C35 specific antiserum or control, pre-bleed BALB / c serum. After a 30 minute incubation, cells were washed twice with staining buffer (PAB) and incubated with FITC-goat anti-mouse IgG (1 μg / sample) for 30 minutes. Samples were washed and analyzed on an EPICS Elite flow cytometer....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| relative frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com