Implantable microelectronic device and method of manufacture
a microelectronic device and manufacturing method technology, applied in the direction of semiconductor devices, semiconductor/solid-state device details, therapy, etc., can solve the problems of imposing a long life requirement on the manner of hermetic sealing, the contact pads of semiconductor devices cannot be simply left exposed, and the structure is generally more difficult to fabrica
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
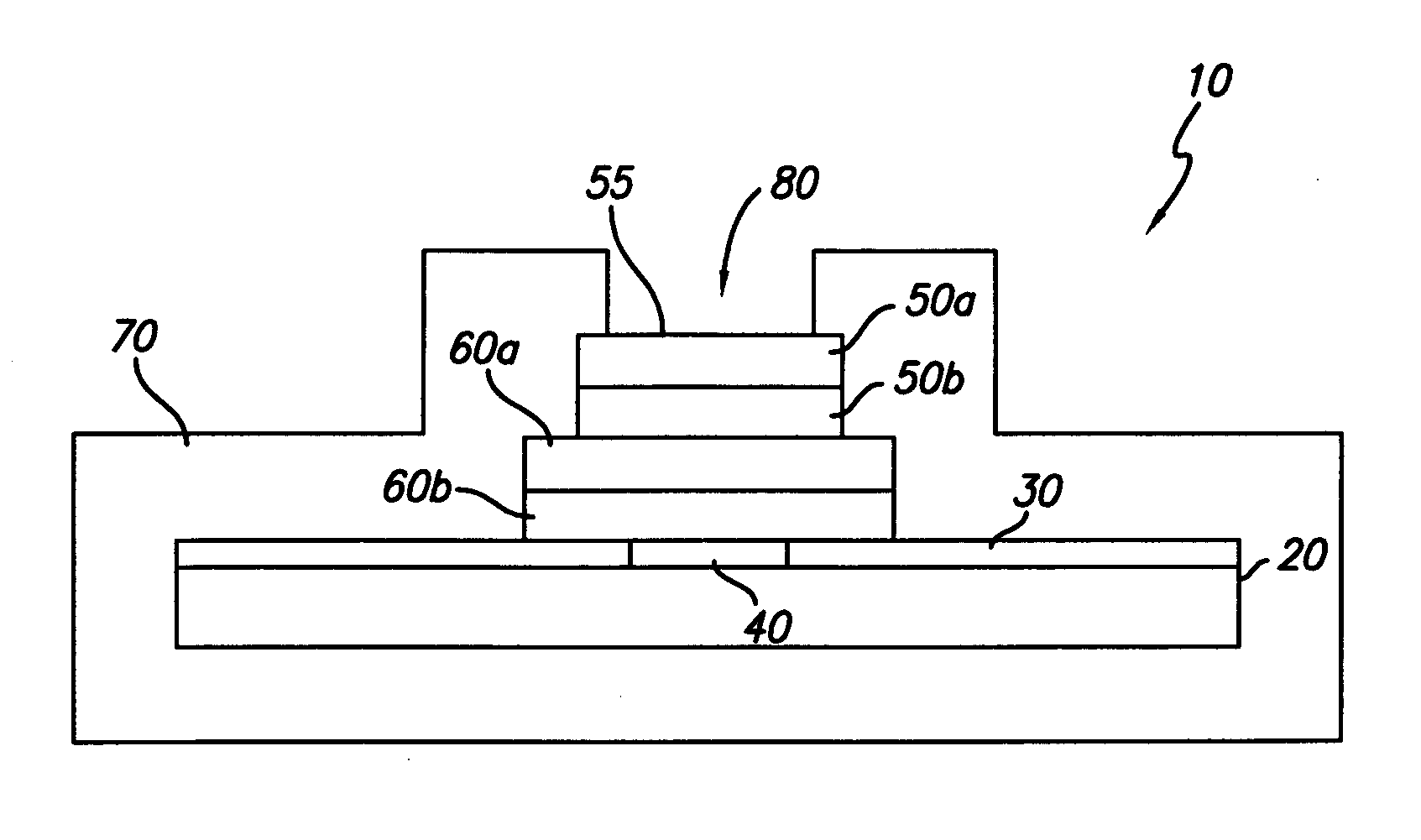
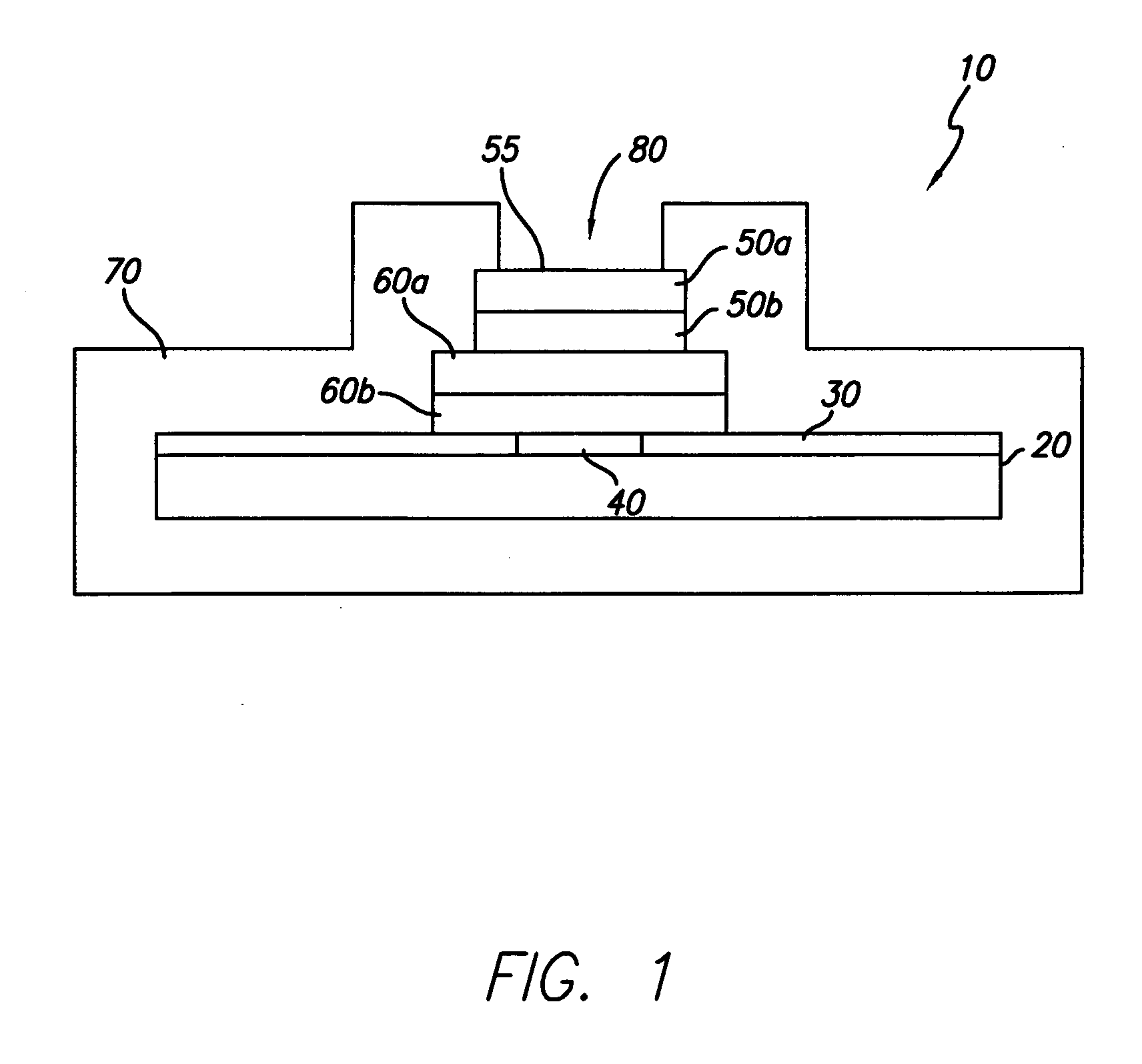
[0024] In general, the present invention is directed to an implantable micro-miniature electronic device, and method of manufacture, with an external electrical contact surface, that has excellent hermetic properties.
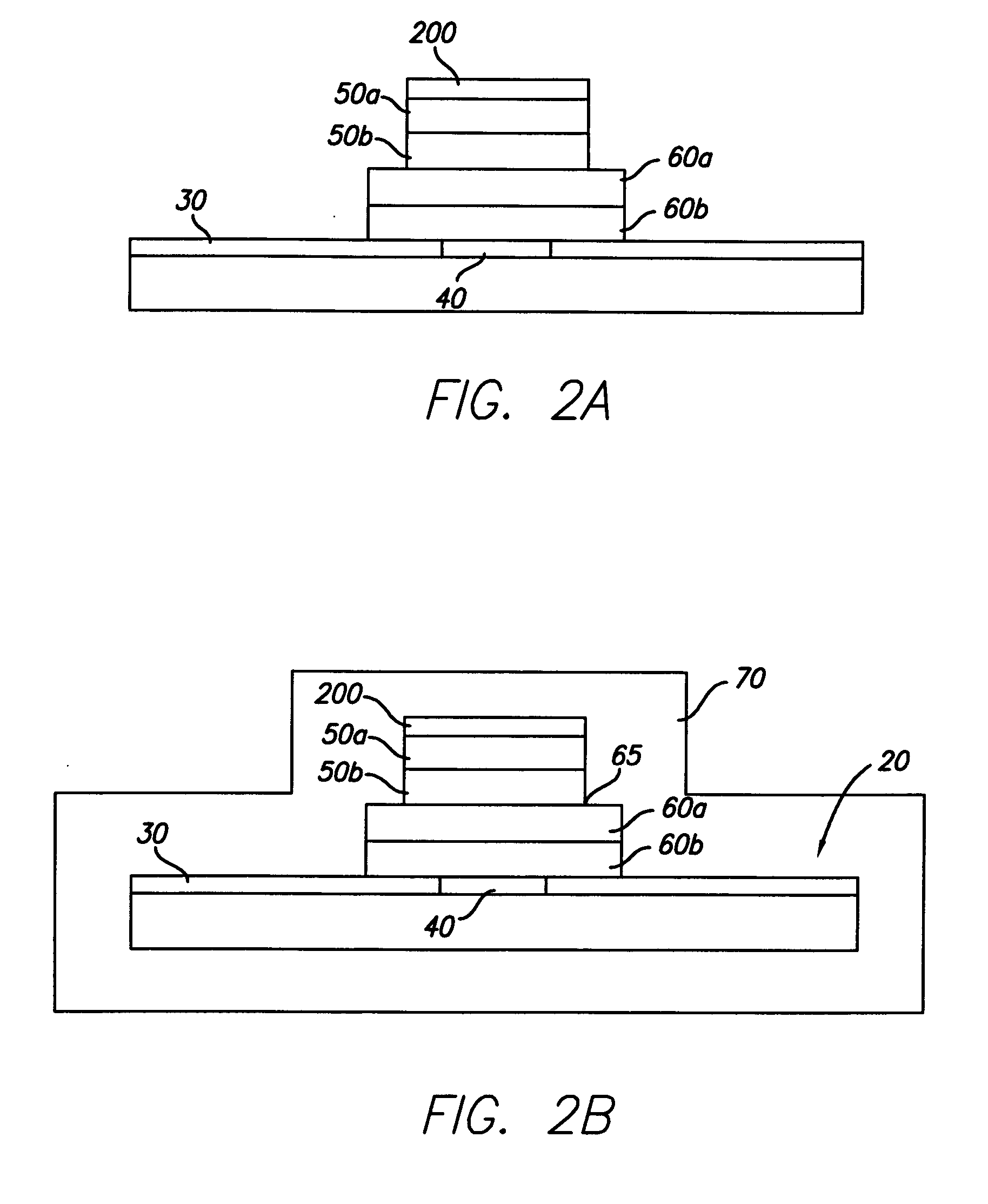
[0025] An exemplary embodiment of an implantable microelectronic device 10 of the present invention is depicted in the simplified, cross-sectional view of FIG. 1. A silicon integrated circuit chip (“IC”) 20 comprises a top electrically insulating (passivation) layer 30 and an electrical contact pad 40 which extends through layer 30 to the surface of IC 20. While the exemplary embodiment of the invention is described in conjunction with IC 20, the invention is also useful with other types of microelectronic devices, whether or not they are fabricated on silicon, which require a hermetic electrical connection. Therefore, the present invention should not be viewed as restricted to use with IC chips.
[0026] While only one contact pad 40 is shown in FIG. 1, those skilled in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com