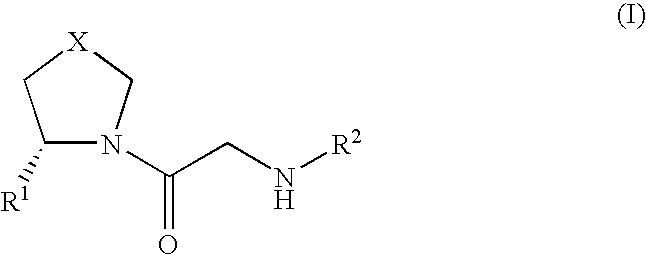
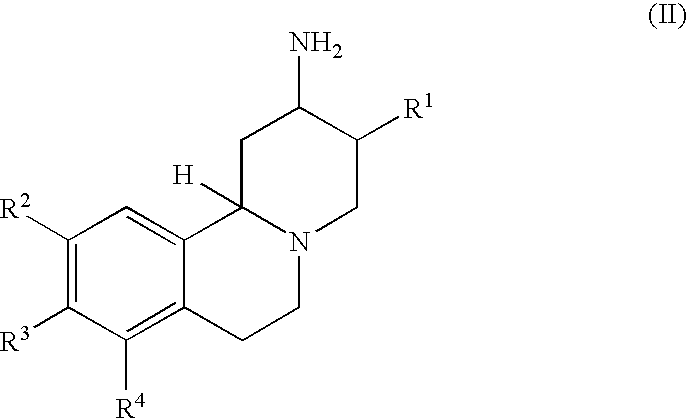
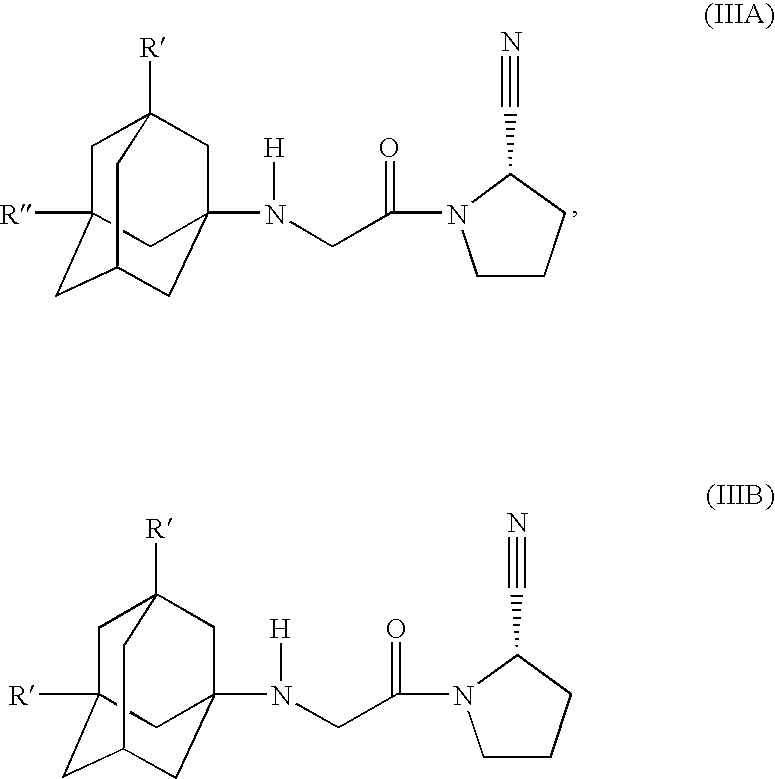
Modified release compositions for DPP-IV inhibitors
a technology of dppiv inhibitors and compositions, which is applied in the direction of peptide sources, extracellular fluid disorders, metabolic disorders, etc., can solve the problems of all three classes of compound having side effects, a major threat to the health of the citizens of the western world, and difficulty in controlling type i diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0410] Coated tablets with the compositions shown in the table below are made according to standard procedures. The specific DPP-IV inhibitor mentioned in the table can be replaced by other DPP-IV inhibitors mentioned above.
Descrip-100 mg200 mg400 mgComponenttiontablettablettabletGranulate(2S)-1-{[2-DPPIV128.4mg256.8mg513.6mg(5-Methyl-2-inhibitorphenyl-oxazol-4-yl)-ethylamino]-acetyl}-pyrrolidine-2-carbonitrilemesylateMicrocrystallineFiller56.4mg112.80mg225.6mgCellulose(Avicel PH-101)Sodium stearylGlidant0.9625mg1.925mg3.85mgfumarateKernel(externel phase)TalcAnti-6mg9mg12mgadhesiveSodium stearylGlidant / 2mg3mg4mgfumarateLubricantCoatOpadryFilm9.50mg15.00mg30.00mgformerEudragit S 100Coat15mg25mg50mgTotal:217mg425mg850mg
example 2
[0411] Coated capsules with the compositions shown in the table below are made according to standard procedures. The specific DPP-IV inhibitor mentioned in the table can be replaced by other DPP-IV inhibitors mentioned above.
50 mg150 mgComponentDescriptioncapsulecapsuleGranulate(2S)-1-{[1,1-Dimethyl-DPP-IV50mg150mg3-(4-pyridin-3-yl-inhibitorimidazol-1-yl)-propylamino]-acetyl}-pyrrolidine-2-carbonitrileMicrocrystallineFiller56.4mg112.80mgCellulose(Avicel PH-102)Externel phaseTalcAnti-1.925mg3.85mgadhesiveSodium stearyl fumarateGlidant / 4.8125mg9.625mgLubricantCapsuleEudragit S:Eudragit25mg40mgL 25:75
example 3
[0412] Capsules with coated pellets with the compositions shown in the table below are made according to standard procedures. The specific DPP-IV inhibitor mentioned in the table can be replaced by other DPP-IV inhibitors mentioned above.
50 mg150 mgComponentDescriptioncapsulecapsuleGranulate(S)-1-((2S,3S,11bS)-2-DPP-IV50mg150mgAmino-9,10-dimethoxy-inhibitor1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-oneMicrocrystallineFiller60mg80mgCellulose(Avicel PH-102)Pregelatinized starchBinder3050Externel phaseTalcAnti-1.925mg3.85mgadhesiveMagnesium stearateGlidant / 4.8125mg9.625mgLubricantCoatEudragit L:Eudragit60mg100mgFS 75:25Capsule
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com