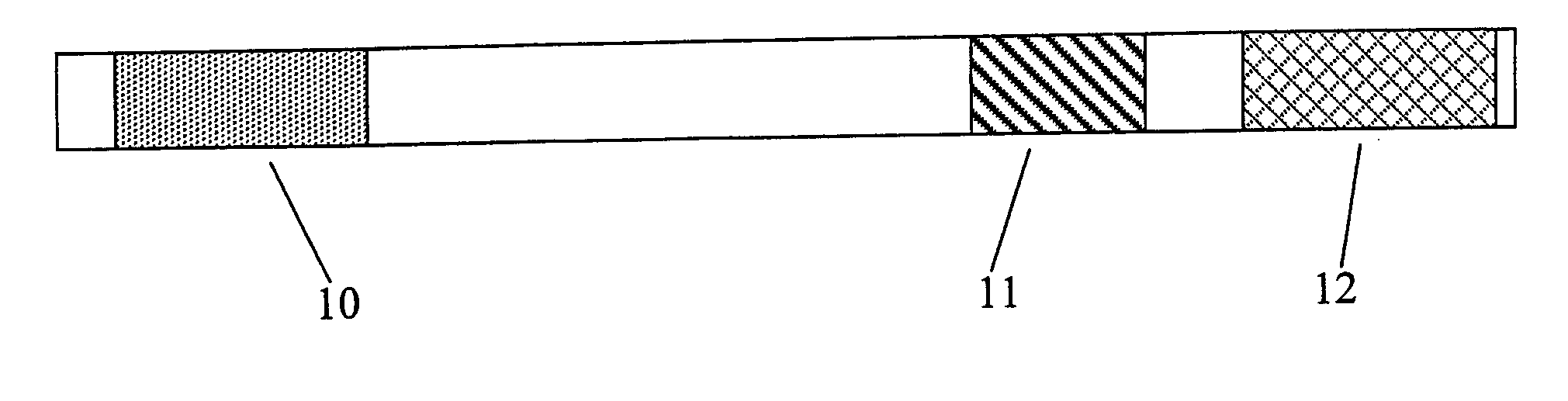
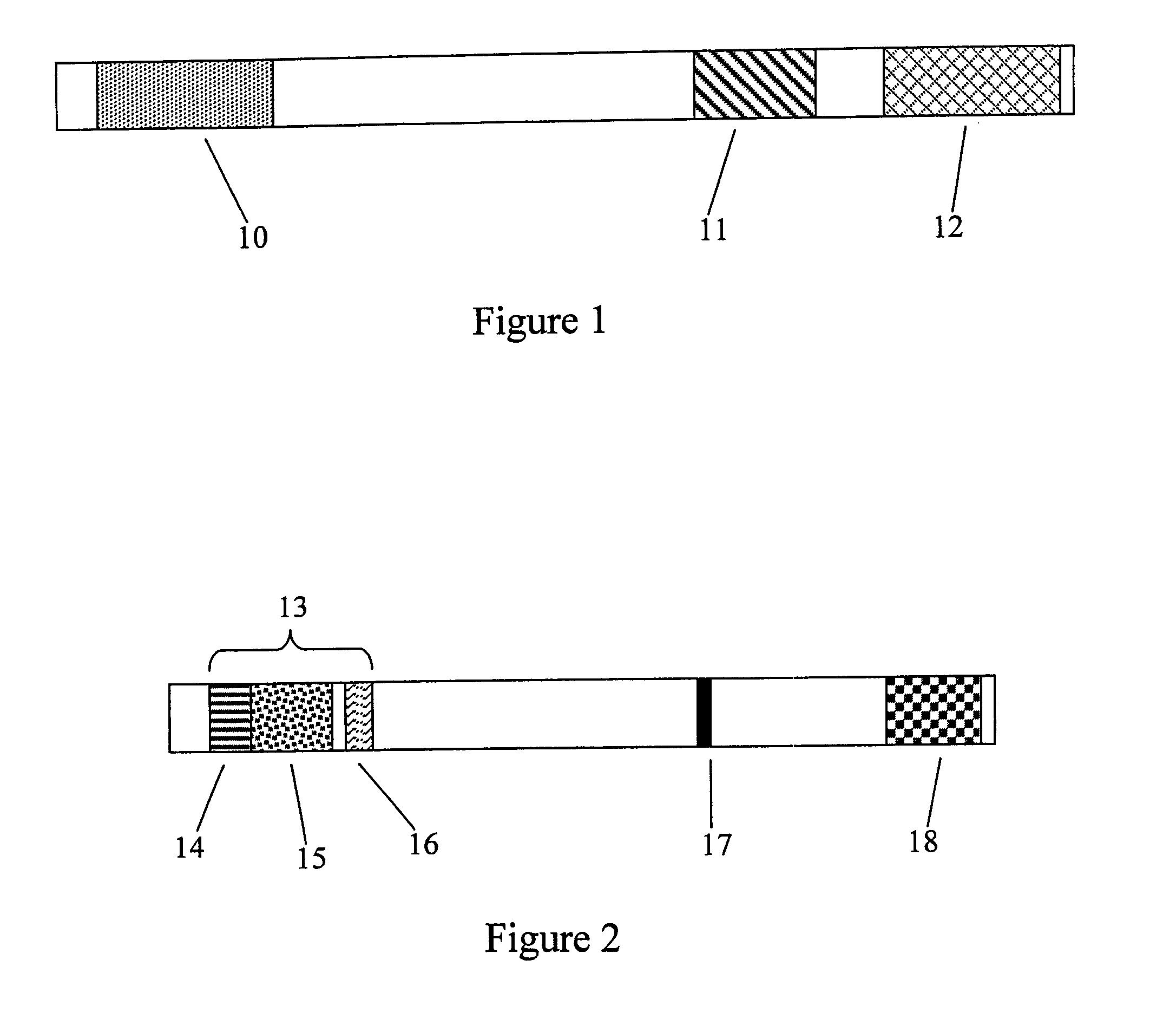
Therapeutic regimen for treating cancer
a cancer and therapy regimen technology, applied in the field of cancer therapy regimens, can solve the problems of limited success of clinicians, inability to effectively treat cancer, and inability to meet the needs of patients,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0092] This example demonstrates the safety and efficacy of the pharmaceutical composition of the present invention comprising (i) a pharmaceutically acceptable carrier and (ii) an adenoviral vector comprising a nucleic acid sequence encoding TNF-α operably linked to a radiation-inducible promoter, wherein the dose comprises from about 4×109 to about 4×1011 particle units (pu) of adenoviral vector, in a clinically-relevant animal model.
[0093] Preclinical toxicology studies were performed in nude mice (nu / nu) bearing a human SQ-20B squamous cell carcinoma zenograft (n=80) and Balb / c mice (n=100). Ten nu / nu mice / sex / group in four groups totaling 80 mice were used in the study. The four groups consisted of: 1) vehicle control, 2) vehicle control plus radiation, 3) pharmaceutical composition comprising 4×109 pu of adenoviral vector plus radiation, and 4) pharmaceutical composition comprising 4×1010 pu of adenoviral vector plus radiation. Doses of vehicle or pharmaceutical composition c...
example 2
[0098] This example illustrates use of the inventive method to therapeutically treat cancer in a human as indicated by size reduction of tumor mass.
[0099] Patients with solid tumors accessible for repeated intratumoral injections were selected for treatment using the inventive method. These patients had failed one or more prior therapies. Patients were injected intra-tumorally with a pharmaceutical composition comprising one of five dose levels of the TNF-α coding sequence-containing adenoviral vector described in Example 1 (4×109-4×1011 pu in ½ log increments) over a maximum 6-week therapeutic period. Several patients comprised a lesion treated by radiation only, which served as a control. A single dose of pharmaceutical composition was administered via multiple injections to the tumor on day 1 and day 4 of weeks 1 and 2 of the therapeutic period, and once weekly for weeks 3-6. For each dose, the multiple injections were administered in a pattern such that the injections were equa...
example 3
[0103] This example illustrates use of the inventive method to reduce the size of a tumor associated with soft tissue sarcoma.
[0104] Patients with extremity soft tissue sarcomas are selected for treatment using the present inventive method. Patients are injected intratumorally with a pharmaceutical composition comprising one of five dose levels of the TNF-α coding sequence-containing adenoviral vector described in Example 1 (4×109-4×1012 pu in 1-½ log increments) over a 5-week therapeutic period. A single dose of pharmaceutical composition is administered via multiple injections to the tumor on day 1 and day 4 of week 1 of the therapeutic period, and once weekly for weeks 2-5. For each dose, the multiple injections are administered to the tumor in parallel lines. Concomitant radiation therapy is initiated in week 1 and administered for five consecutive days, and not administered for two days, for each week of the therapeutic period, achieving a total dose of 30-70 Gy.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com