Chamber for reaction of lithium and deuterium
a chamber and lithium-deuterium technology, applied in the field of electrochemical devices, can solve the problems that the aqueous electrolyte is not the only appropriate solution, and achieve the effect of facilitating interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] A description of preferred embodiments of the invention follows.
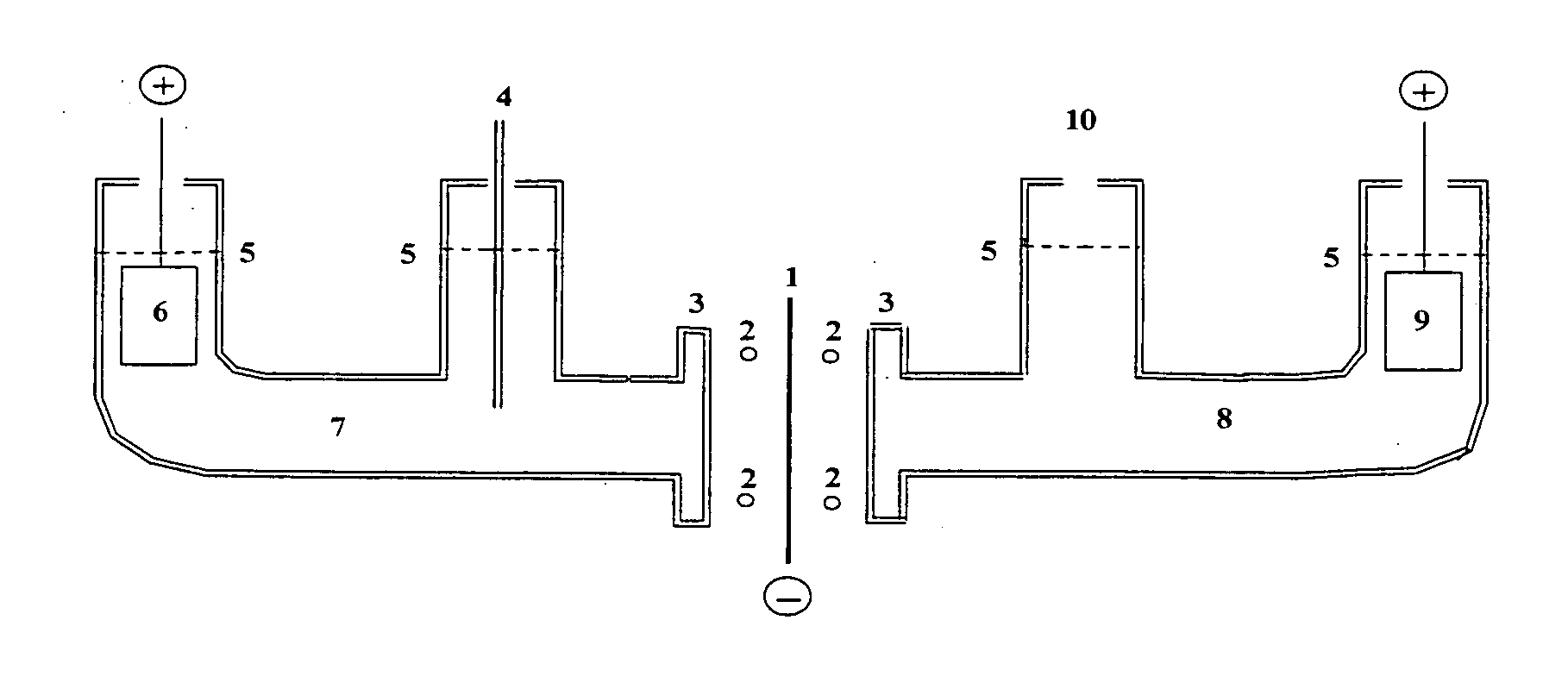
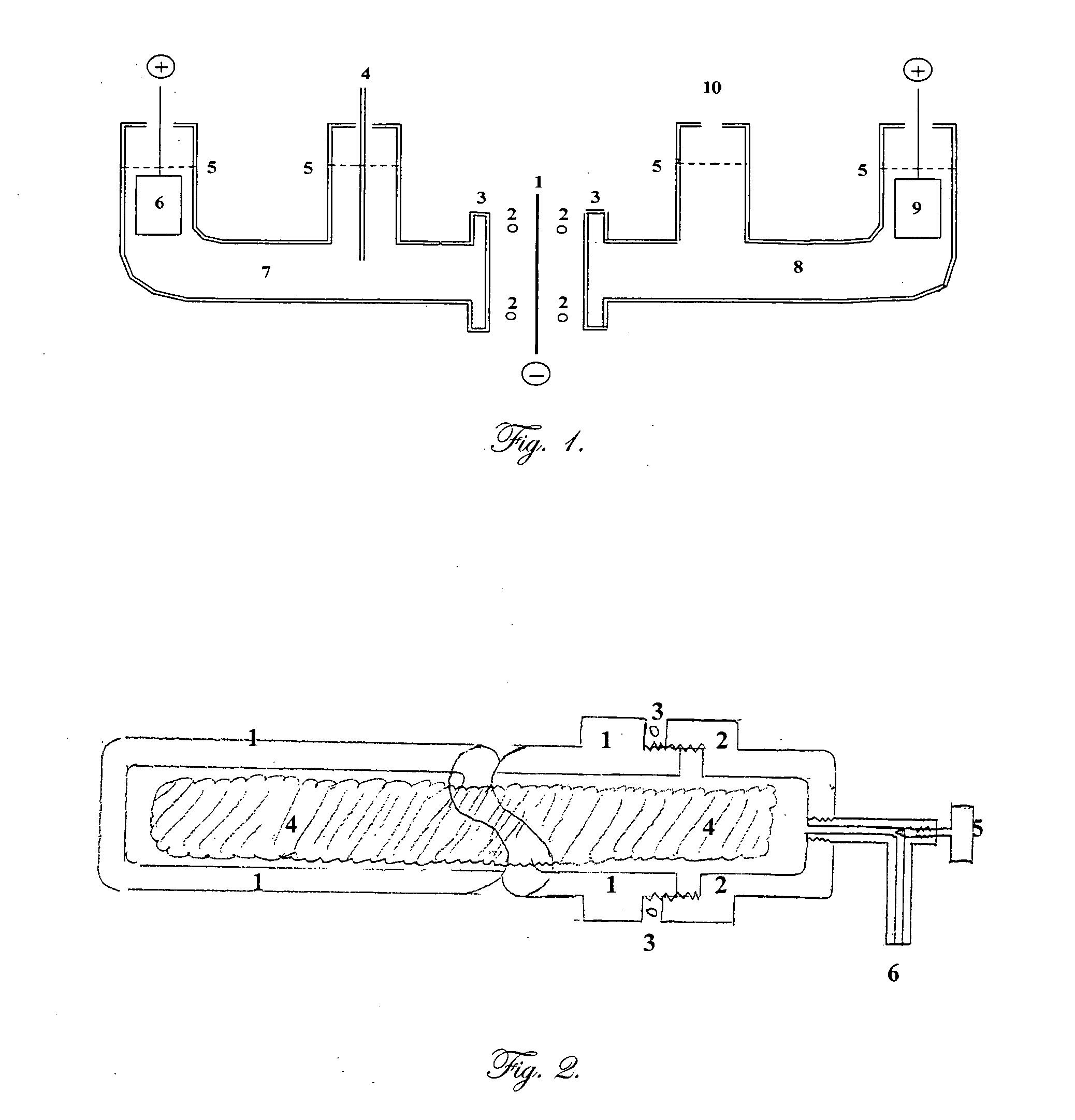
[0019]FIG. 1 is a cross section of an electrochemical cell according to one embodiment of this invention. The body is constructed from cylindrical borosilicate glass tubes, such as Pyrex™. Element number 1 is a flat membrane, for example palladium foil, in which the hydrogen (deuterium) and the lithium are to be brought together. It is the common cathode, or the negative electrode where electrochemical reduction occurs. This electrode is connected to the negative terminal of each of the two direct current power supplies. The power supplies regulate the current through each half of the cell.
[0020] Element number 2 shows the position of the O ring seals which fit within flanges, 3, on each half of the cell. Not shown is the clamp needed to compress the flanges against the O rings and the common cathode, 1.
[0021] During electrolysis in the aqueous (left) side, some, but not all of the hydrogen (deuterium) will be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| metallic | aaaaa | aaaaa |
| heat resistant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com