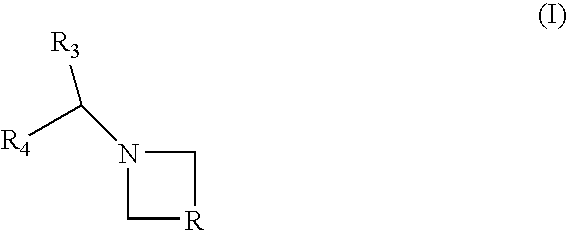
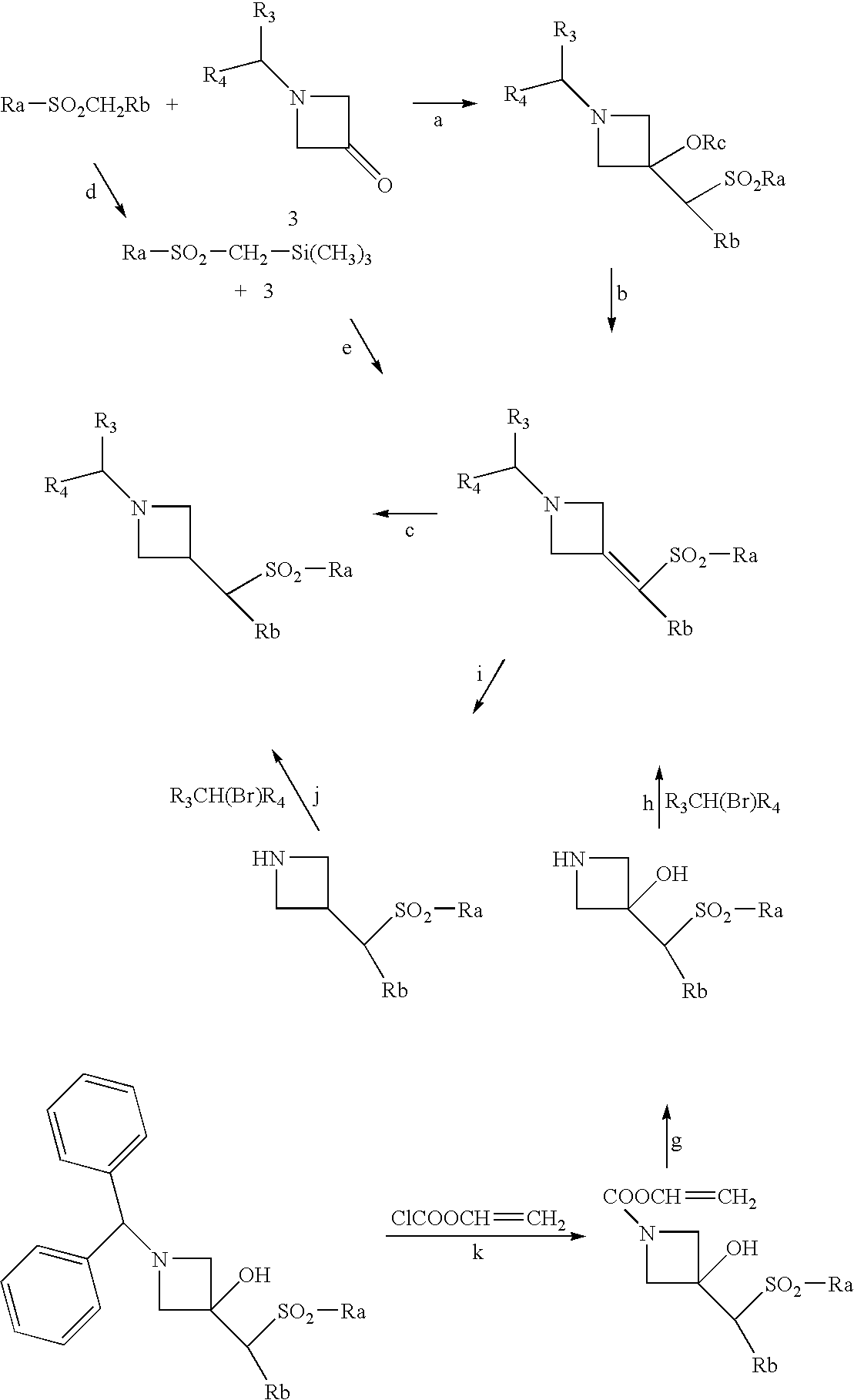
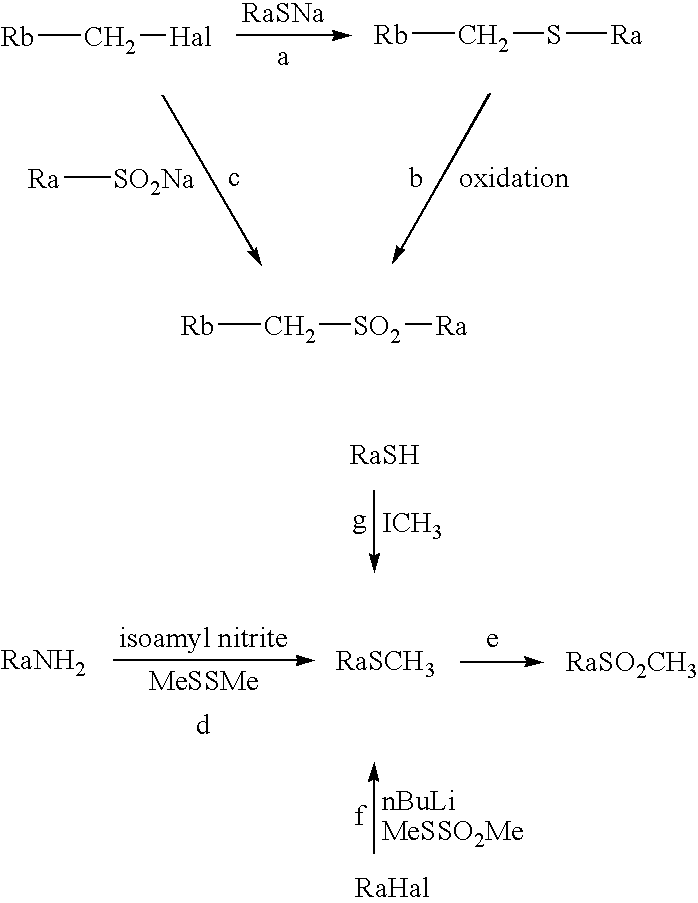
Combination of a cb1 receptor antagonist and of a product which activates dopaminergic neurotransmission in the brain, the pharmaceutical compositions comprising them and their use in the treatment of parkinson's disease
a technology of cb1 receptor and product, which is applied in the field of combination of a cb1 receptor antagonist and a product which activates dopaminergic neurotransmission in the brain, can solve the problems of significant side effects, wear-off effect, and decrease in effectiveness, and achieve the effect of reducing side effects and potentiating symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0406] N-{1-[Bis(4-chlorophenyl)methyl]azetidin-3-yl}-N-(pyrid-3-yl)methylsulfonamide can be prepared by carrying out the preparation in the following way: 0.042 cm3 of phosphorus trichloride is added to a solution of 0.144 g of N-{1-[bis(4-chlorophenyl)-methyl]azetidin-3-yl}-N-(1-oxidopyrid-3-yl)methylsulfonamide in 5 cm3 of chloroform and then the mixture is heated to the reflux temperature. After stirring for 1 hour 30 minutes, the reaction mixture is allowed to return to normal temperature, 5 cm3 of 0.1N hydrochloric acid are then added to the mixture, and then the mixture is stirred and separated by settling. The organic phase is diluted with 20 cm3 of chloroform, dried over magnesium sulfate, filtered and then concentrated to dryness under reduced pressure (2.7 kPa). The residue is chromatographed on a column of silica gel (particle size 0.063-0.200 mm, height 9 cm, diameter 1.8 cm), elution being carried out under a pressure of 0.1 bar of argon with a mixture of dichlorometha...
example 2
Method 1:
[0407] N-{1-[Bis(4-chlorophenyl)methyl]azetidin-3-yl}-N-(3,5-difluorophenyl)methylsulfonamide can be prepared by carrying out the preparation in the following way: 1.0 g of cesium carbonate is added to a mixture of 1.23 g of 1-[bis(4-chlorophenyl)methyl]-azetidin-3-yl}methylsulfonate and of 0.66 g of N-(3,5-difluorophenyl)methylsulfonamide in 25 cm3 of dioxane. After stirring for 5 hours at the reflux temperature and then for 20 hours at 20° C., 50 cm3 of diethyl ether and 30 cm3 of brine are added to the reaction mixture and then the reaction mixture is stirred and separated by settling. The organic phase is dried over magnesium sulfate, filtered and then concentrated to dryness at 50° C. under reduced pressure (2.7 kPa). The orange oil obtained is chromatographed on a column of silica gel (particle size 0.040-0.063 mm, height 25 cm, diameter 2.0 cm), elution being carried out under a pressure of 0.5 bar of argon with a mixture of cyclohexane and of ethyl acetate (65 / 35 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com