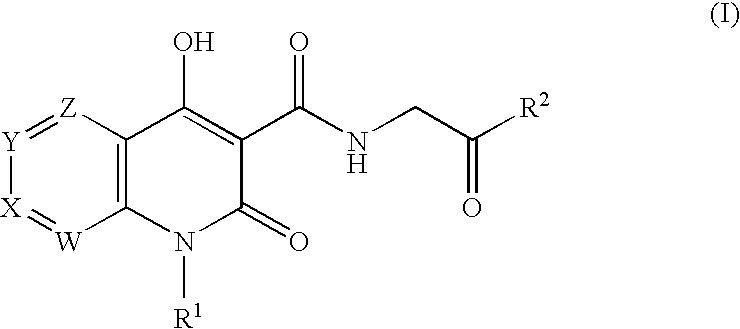
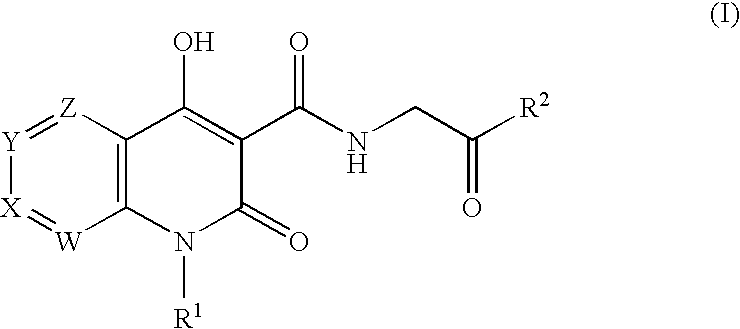
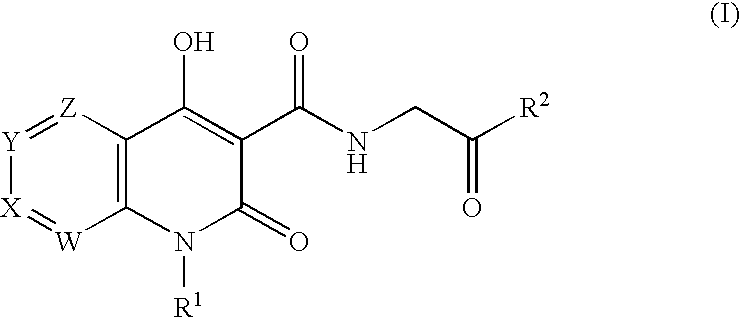
Prolyl Hydroxylase Inhibitors
a technology of prolyl hydroxylase and inhibitor, which is applied in the direction of biocide, drug composition, extracellular fluid disorder, etc., can solve the problems of reduced oxygen levels in the blood, ubiquitination of hif-alpha and subsequent degradation, and achieve the effect of increasing the production of erythropoietin and epo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0088]
N-{[1-(2-Cyclopropylethyl)-4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridin-3-yl]carbonyl}glycine
[0089]A mixture of 1-(2-cyclopropylethyl)-2H-pyrido [2,3-d][1,3]oxazine-2,4(1H)-dione (Prepared according to PCT Int. Appl. (2003), WO 2003059356 A2)(0.232 g, 1.00 mmol) and diethylmalonate (0.152 mL, 1.00 mmol) was treated with 1,8-diazabicyclo[5.4.0]undec-7-ene (0.300 mL, 2.00 mmol). 1,4-dioxane (1.0 mL) was added and the solution was heated to 150° C. for 20 min. in a Biotage Initiator microwave synthesizer (http: / / www.biotage.com). Following cooling, glycine (0. 113 g, 1.50 mmol) was added and the solution was heated to 200° C. for 20 min. in a Biotage Initiator microwave synthesizer. The reaction mixture was then cooled, treated with 6M aqueous sodium hydroxide (2.0 mL), diluted with water and extracted with diethyl ether. The aqueous layer was then acidified with 6M aqueous hydrochloric acid and extracted twice with ethyl acetate. The organic solution was dried over MgSO4, filte...
example 2
[0090]
N-{[4-hydroxy-1-(3-methylbutyl)-2-oxo-1,2-dihydro-1,8-naphthyridin-3-yl]carbonyl}glycine
2a) Ethyl 2-[(3-methylbutyl)amino]-3-pyridinecarboxylate
[0091]A mixture of ethyl-2-chloronicotinic carboxylate (2.00 g, 10.8 mmol) and 3-(methylbutyl)amine (1.88 mL, 16.1 mmol) in ethanol (3.0 mL) was heated to 180° C. for 40 min. in a Biotage Initiator microwave synthesizer. The mixture was added to a solution of saturated aqueous sodium bicarbonate and extracted twice with ethyl acetate. The combined organic portions were dried over magnesium sulfate, filtered, and concentrated in vacuo. The residue was purified via flash column chromatography (60% ethyl acetate in hexanes) to afford the title compound as a clear oil (2.05 g, 80%). 1H NMR (400 MHz, CHLOROFORM-d) δ ppm 8.30 (dd, J=4.7, 1.9 Hz, 1 H), 8.13 (dd, J=7.6, 2.0 Hz, 1 H), 7.94 (br. s., 1 H), 6.51 (dd, J=7.8, 4.8 Hz, 1 H), 4.33 (q, J=7.2 Hz, 2 H), 3.48-3.58 (m, 2 H), 1.68-1.82 (m, 1 H), 1.51-163 (m, 2 H), 1.39 (t, J=7.2 Hz, 3 H), 0...
example 3
[0094]
N-{[1-(3,3-dimethylbutyl)-4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridin-3-yl]carbonyl}glycine
3a) Ethyl 2-[(3,3-dimethylbutyl)amino]-3-pyridinecarboxylate
[0095]A mixture of ethyl-2-chloronicotinic carboxylate (0.518 g, 2.79 mmol) and (3,3-dimethylbutyl)amine (0.560 mL, 4.18 mmol) in ethanol (3.0 mL) was heated to 180° C. for 40 min. in a Biotage Initiator microwave synthesizer. The mixture was concentrated and the residue was diluted in water, treated with saturated aqueous sodium bicarbonate, and extracted with ethyl acetate. The combined organic portions were dried over magnesium sulfate, filtered, and concentrated in vacuo. The residue was purified via flash column chromatography (60% ethyl acetate in hexanes) to afford the title compound as a clear oil (0.655 g, 94%). 1H NMR (400 MHz, CHLOROFORM-d) δ ppm 8.31 (dd, J=4.8, 2.0 Hz, 1 H), 8.12 (dd, J=7.6, 2.0 Hz, 1 H), 7.87 (br. s., 1 H), 6.51 (dd, J=7.6, 4.8 Hz, 1 H), 4.33 (q, J=7.2 Hz, 2 H), 3.30-3.61 (m, 2 H), 1.55-1.68 (m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com