Compositions and methods for inhibiting tumor growth and metastasis
a technology of plasminogen and cell surface receptor, which is applied in the field of modulating the activity of p11 protein, can solve the problem of rate-limiting binding of plasminogen to its cell surface receptor, and achieve the effect of reducing the production of a61
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Stimulation of A61 Production
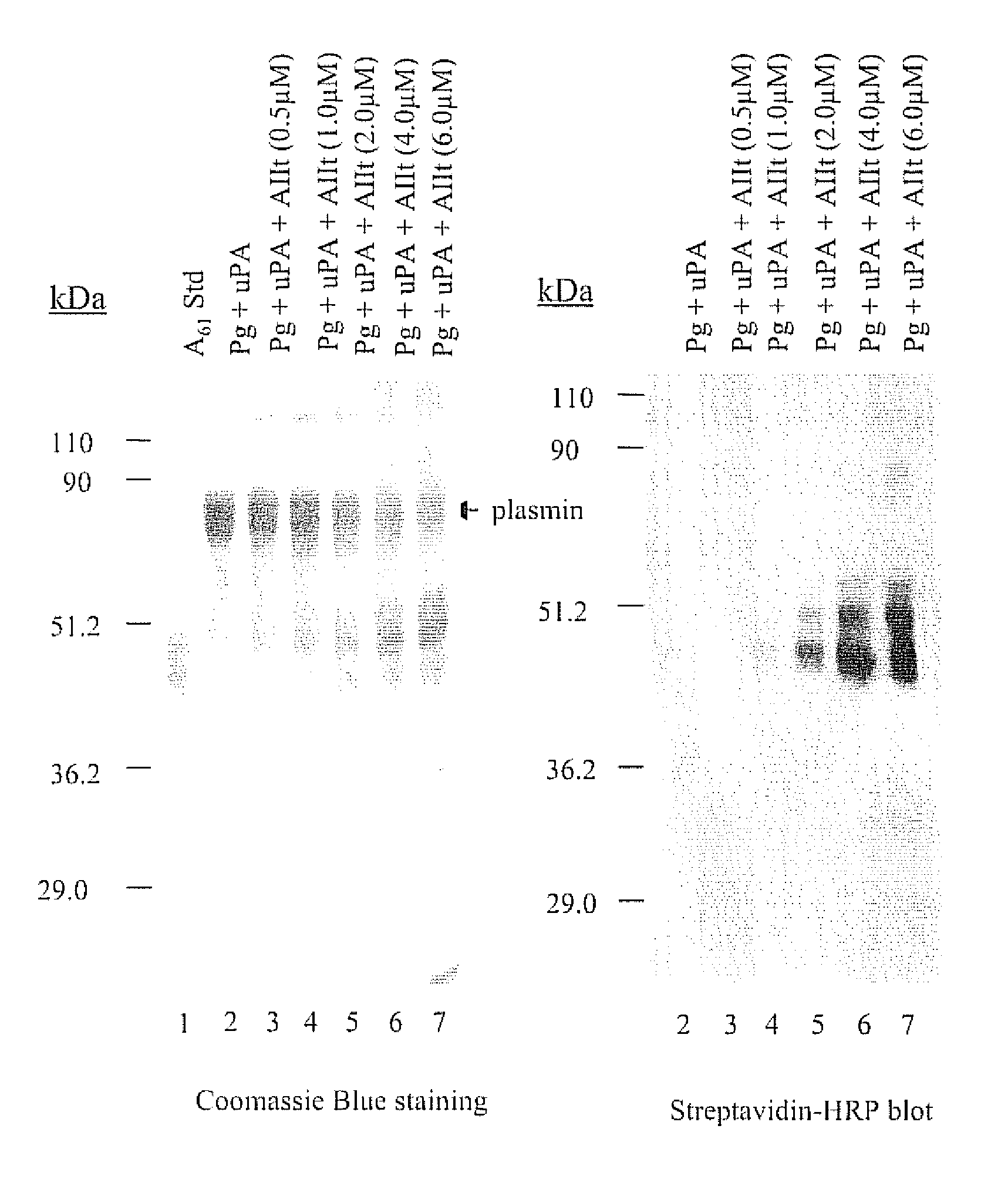
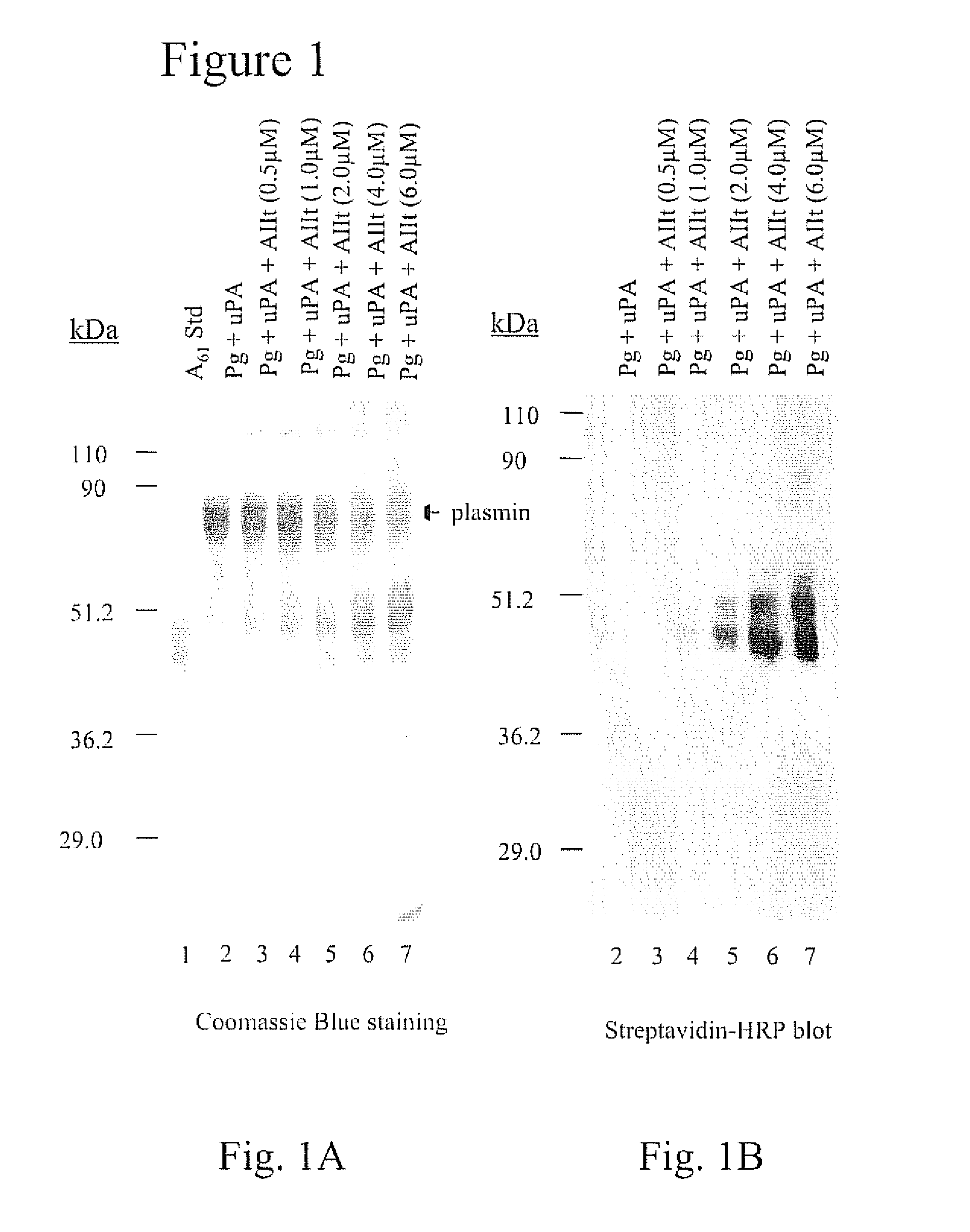
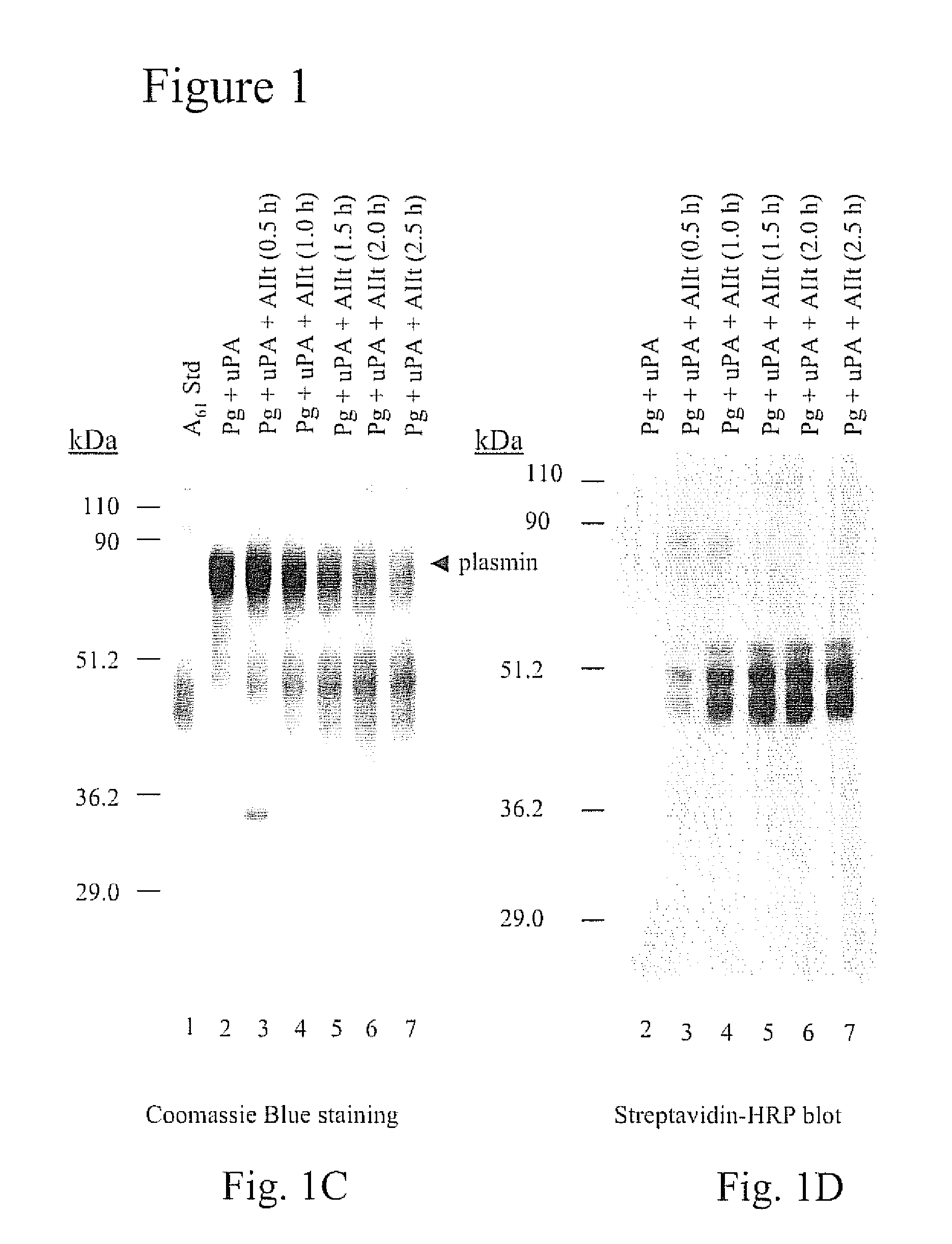
[0091] A61 is an internal fragment of plasminogen that encompasses the sequence Lys78-Lys468 (SEQ ID NO:1). The release of A61 from plasmin is facilitated by the reduction of the Cys462-Cys541 disulfide bond of plasmin. Therefore, the release of A61 generates a free sulfhydryl residue at Cys462. Since plasminogen and plasmin contain only disulfides, A61 can be discriminated from these proteins on the basis of its reactivity with free sulfhydryl-reactive reagents such as 3-(N-maleimidylpropionyl)biocytin (MPB). The reaction of free-sulfhydryl-containing proteins with MPB results in the biotinylation of the protein which allows easy detection with streptavadin-HRP.
[0092] As shown in FIG. 1A (lane 2), the incubation of u-PA with plasminogen resulted in the generation of plasmin. As expected, the plasmin generated by this reaction did not contain a free cysteinyl residue and therefore did not react with MPB (FIG. 1B, lane 2). However, the addition of AIIt ...
example 2
Stimulation of A61 Production by Other Disulfide Reductases
[0100] Protein disulfide isomerase, thioredoxin and phosphoglycerate kinase are three protein disulfide reductases that are secreted by cultured cells (Lay et al., 2000, Nature 408:869-873; Rubartelli et al., 1992, J Biol Chem 267:24161-24164; Terada et al., 1995, J Biol Chem 270:20410-20416; Soderberg et al., 2000, Cancer Res 60:2281-2289). The three reductases have been shown to act as plasmin reductases (Stathakis et al., 1997, J Biol Chem 272:20641-20645; Lay et al., 2000). As shown in FIG. 6, under the assay conditions AIIt was a more potent plasmin reductase than the other reductases in vitro.
[0101] Thioredoxin and protein disulfide isomerase share a common sequence, Trp-Cys-Gly-Pro-Cys-Lys (SEQ ID NO:4), which participates in the cleavage, formation and reshuffling of disulfide bonds. This sequence is not present in phosphoglycerate kinase or AIIt, suggesting that these reductases have distinct catalytic mechanisms....
example 3
Down-Regulation of AIIt Blocks A61 Generation
[0102] HT1080 fibrosarcoma cells were stably transfected (transduced) with a pLin retroviral vector encoding a p11 gene in the sense (pLin-p11S) or antisense (pLin-p11AS) orientation, or an empty pLin vector (pLin-V). The pLin-p11AS transduced cells showed a decrease in both p11 and p36 subunits on the cell surface whereas pLin-p11S transduced cells showed an increase in both p11 and p36 subunits (FIG. 7A). As shown in FIG. 7B, incubation of the pLin-p11S transduced cells with plasminogen resulted in enhanced A61 formation compared to the pLin-V control cells. In contrast, the pLin-p11AS transduced cells failed to produce A61. Additionally, HeLa cells transfected with a p11 antisense expressing vector also failed to convert plasminogen to A61.
[0103] The data in FIG. 7B establishes a role for AIIt in A61 formation in HT 1080 fibrosarcoma cells. However, it is unclear if plasmin could be directly reduced or if plasmin autoproteolysis prec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com