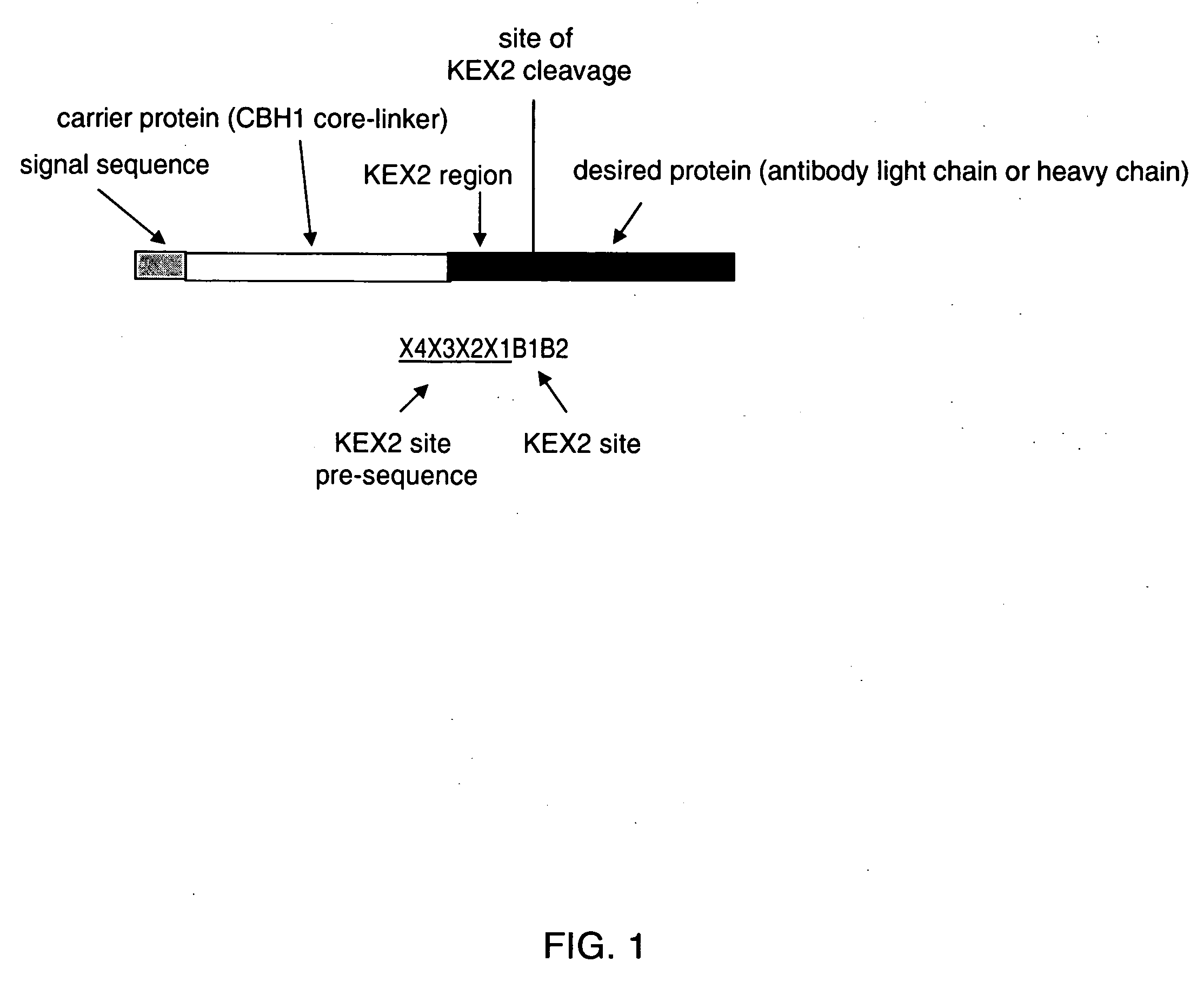
KEX2 cleavage regions of recombinant fusion proteins
a fusion protein and kex2 technology, applied in the field of enhanced secretion and can solve the problems of high production protein levels with limited risk of contamination, relative quick scale up time, etc., and achieve enhanced secretion and/or cleavage of desired proteins, the effect of identifying enhanced secretion and/or cleavag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of a Trastuzumab (Light Chain Expression Strain Containing a KRGGG (SEQ ID NO: 2) KEX2 Cleavage Site
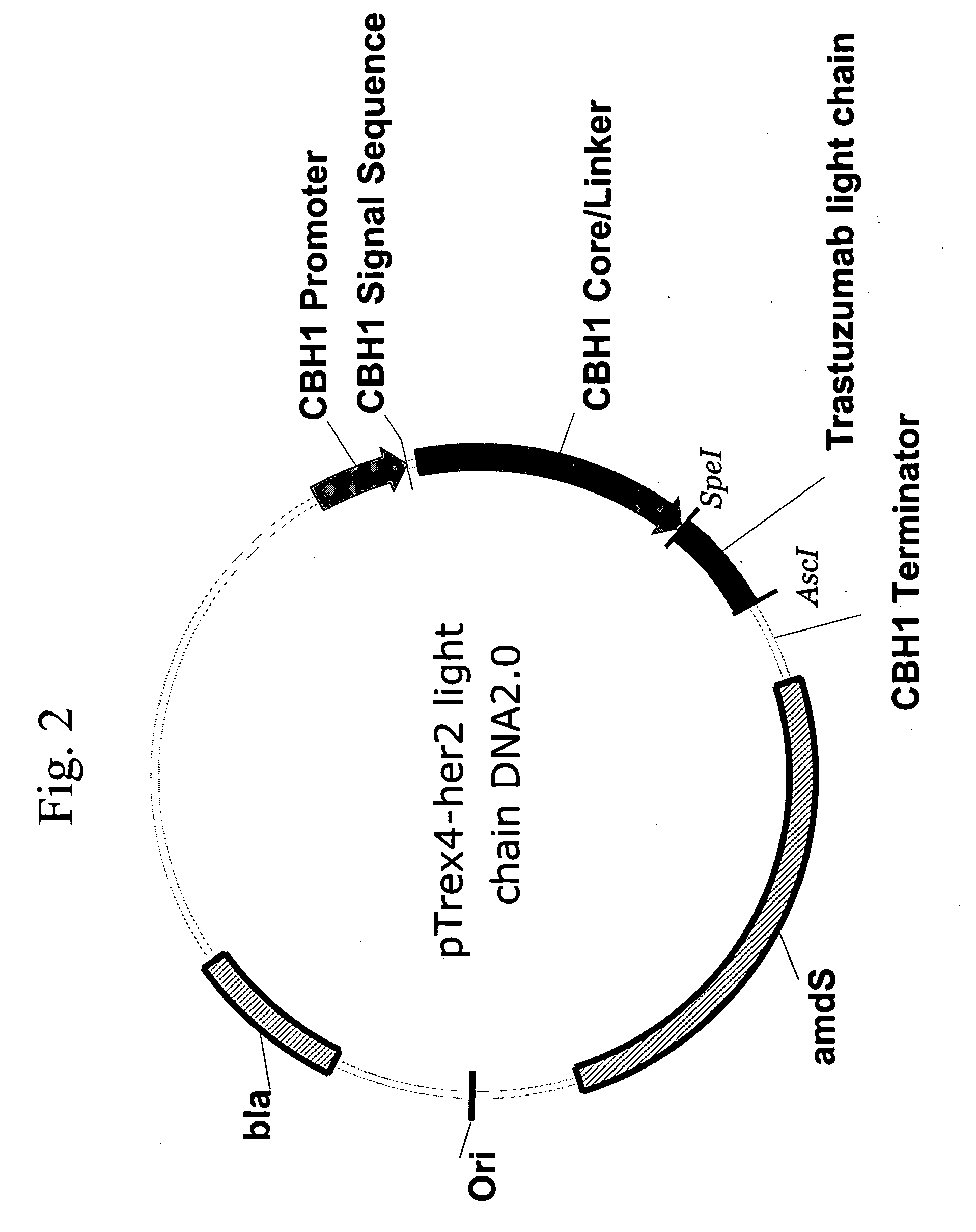
[0169]DNA (SEQ ID NO:1) encoding the light chain of trastuzumab according to the published amino acid sequence of antibody 4D5-8 (Carter et al, Proc. Natl. Acad. Sci. 1992 89: 4285-4289) was synthesized by DNA2.0 Inc. (1455 Adams Drive, Menlo Park, Calif. 94025).
(SEQ ID NO: 1)ACTAGTAAACGCGGTGGCGGTGATATTCAAATGACACAATCTCCTTCTTCTCTGTCAGCCTCAGTGGGCGACCGTGTGACGATTACTTGCCGCGCCTCTCAGGACGTTAACACTGCCGTCGCATGGTACCAGCAGAAGCCAGGCAAGGCGCCCAAGCTTCTGATTTACAGCGCTTCGTTCCTGTACTCTGGCGTGCCATCCCGCTTCTCTGGCAGCCGAAGCGGCACGGATTTCACCCTGACCATTTCGTCCCTGCAGCCCGAGGATTTCGCCACGTATTACTGCCAGCAGCACTACACCACTCCACCCACCTTTGGCCAAGGAACGAGAGTCGAAATCACTCGCACGGTCGCTGCCCCTTCAGTCTTCATCTTCCCCCCCAGCGACGAACAGCTGAAGTCTGGTACGGCCAGCGTCGTTTGCTTGCTTAATAACTTCTATCCGCGAGAGGCGAAGGTCCAATGGAAGGTTGATAACGTTCTGCAGTCCGGCAATTCGCAGGAGAGCGTGACCGAGCAGGATTCAAAGGATAGCACCTACTCACTCAGCAGCACCCTGACGTTGTCCAAGGCCGATTACGAGAAGCATAAGTTGTATGCATGCGAGG...
example 2
Construction of a Trastuzumab Light Chain Expression Strain Containing the GGGKR (SEQ ID NO: 5) KEX2 Cleavage Site
[0171]Two primers (GGACTAGTGGTGGCGGTAAACGCGATATTCAAATGACACAATCT C; SEQ ID NO:3 and AAGGCGCGCCTTAGCACTCGCCTCGATTG; SEQ ID NO:4) were synthesized by Invitrogen (1600 Faraday Avenue. Carlsbad, Calif. 92008) and used to amplify trastuzumab light chain DNA.
[0172]The resulting PCR fragment encodes the antibody light chain containing a GGGKR (SEQ ID NO:5) sequence kex2 site at its N-terminal end. The PCR fragment was digested with restriction enzymes SpeI and AscI and cloned to expression Vector pTrex4 to generate a plasmid named as pTrex4-GGGKR-her2 DNA2.0. Fidelity of the PCR fragment was analyzed by DNA sequencing. The plasmid was digested with XbaI restriction enzyme and transformed biolistically using standard techniques into the T. reesei strain described above. More than 20 transformants were obtained and transferred to new plates. A total of 21 stable transformants were...
example 3
Construction of a Trastuzumab Light Chain Expression Strain Containing a GGGKRGGG (SEQ ID NO: 7) KEX2 Cleavage Site
[0173]Two oligos, GGACTAGTGGCGGTGGCAAACGCGGTGGCGGTGATATTC (SEQ ID NO. 6) and AAGGCGCGCCTTAGCACTCGCCTCGATTG (SEQ ID NO. 4), were synthesized by Invitrogen and used to amplify light chain DNA. The resulting PCR fragment encodes light chain and GGGKRGGG (SEQ ID NO:7) sequence for kex2 cleavage. The PCR fragment was digested with restriction enzymes SpeI and AscI and cloned to expression Vector pTrex4 to generate a plasmid named as pTrex4-GGGKRGGG-her2 light chain DNA2.0. Fidelity of the PCR fragment was analyzed by DNA sequencing. The plasmid was digested with XbaI restriction enzyme and transformed biolistically into the T. reesei strain as described above. More than 10 transformants were obtained and transferred to new plates. 3 stable transformants were selected to grow in Proflo media for 2 days at 30° C. 5 mls of 2 days old culture from Proflo were transferred to 50 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
| catalytic activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com