Use Of 5,6-Dimethylxanthenone-4-Acetic Acid as an Antimicrobial Agent
a technology of dimethylxanthenone and acetic acid, which is applied in the field of use of 5,6-dimethylxanthenone4acetic acid as an antimicrobial agent, can solve the problems of antiviral therapy (and prophylaxis), likely to be toxic to the host, and interfere significantly with viral replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0077]Methods employed are as described by Roberts, Z. J. et al. (2007) (“The Chemotherapeutic Agent DMXAA Potently And Specifically Activates The TBK1-IRF-3 Signaling Axis,” J. Exp. Med. 204(7):1559-1569), and Vogel, S. N. et al. (1987) (“Macrophages From Endotoxin-Hyporesponsive (Lpsd) C3H / Hej Mice Are Permissive For Vesicular Stomatitis Virus Because Of Reduced Levels Of Endogenous Interferon: Possible Mechanism For Natural Resistance To Virus Infection,” J. Virol. 61(3):812-818), except as indicated below.
Cells and Cell Culture
[0078]Thioglycollate-elicited murine peritoneal macrophages were obtained from 5- to 6-wk-old female C57BL / 6J mice (The Jackson Laboratory, Bar Harbor, Me.), IRF-3+ / + and IRF-3− / − mice (Dr. Tadatsugu Taniguchi, University of Tokyo, Tokyo, Japan) and TLR4+ / + and TRL4− / − mice. Murine macrophage-like RAW 264.7 cells and Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection (Manassas, Va.). MEFs fro...
example 2
Antiviral Activity of DMXAA
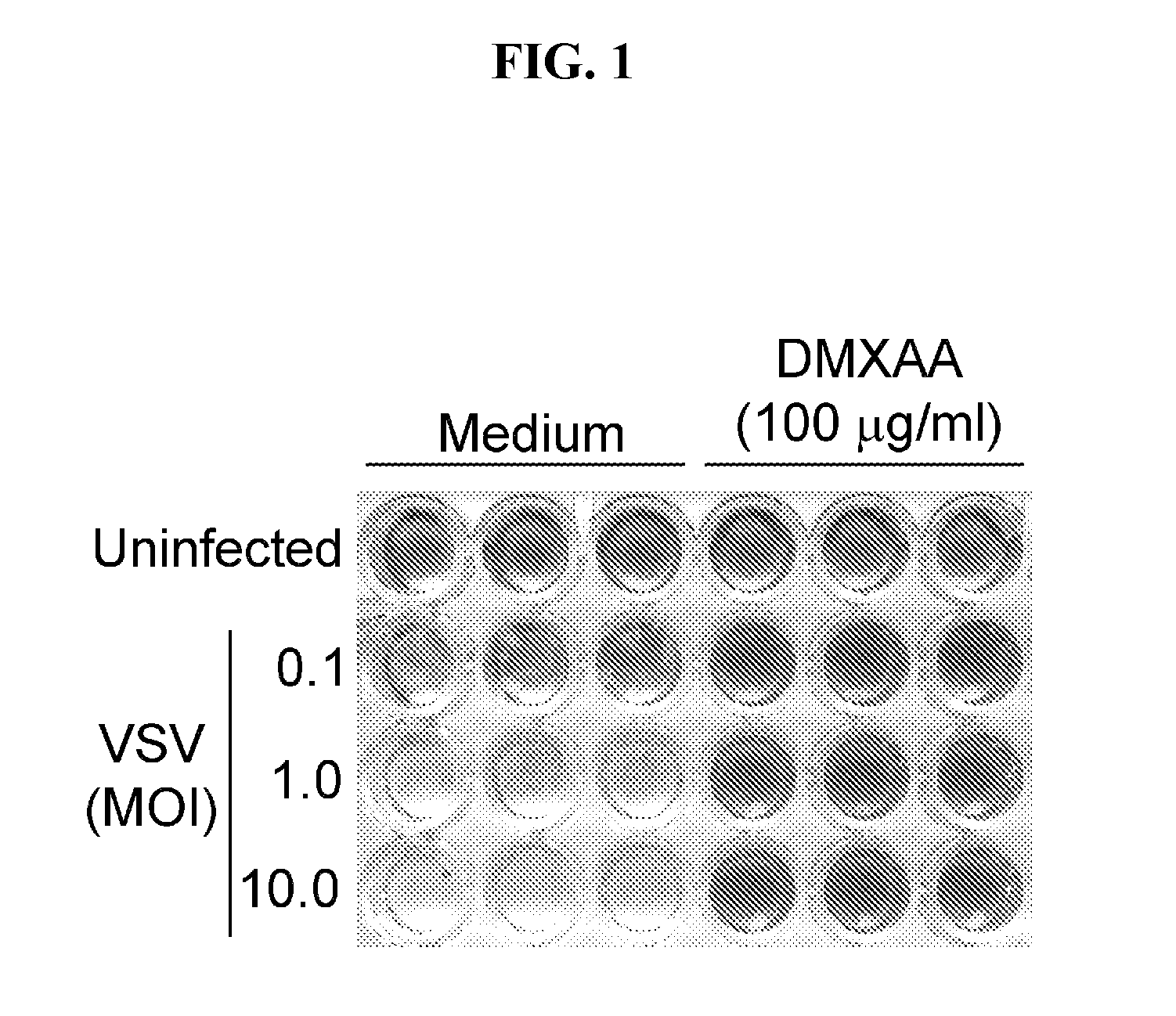
[0089]FIG. 1 shows that exposure of untreated RAW 264.7 macrophages to increasing titers of VSV results in increasing levels of viral infection and, consequently, increasing CPE (i.e., lysis of macrophages as evidenced by decreasing staining with crystal violet). In contrast, pretreatment of the macrophages for 6 h with 100 μg / ml of DMXAA completely prevents VSV infection and CPE at even the highest level of exposure to VSV (i.e., MOI=10.0).
[0090]TABLE 1 demonstrates that DMXAA inhibits the CPE of influenza virus on MDCK cells. The IC50s for DMXAA's direct and indirect antiviral activities are 5.0 μg / ml and 2.0 μg / ml, respectively.
TABLE 1Inhibition Assay for DMXAA Against Influenza / A / Wuhan[ ] Test Compound per mlDirect (CPE)Indirect (CPE)100 ug DMXAA++++++++25 ug DMXAA++++++++6.25 ug DMXAA++++++++1.56 ug DMXAA+−+−+++−.39 ug DMXAA−−−−−−+−.01 ug DMXAA−−−−−−−−.024 ug DMXAA−−−−−−−−.0006 ug DMXAA−−−−−−−−Tamiflu (0.1 ug)−+++Relenza (0.12 ug)−+++Virus Control−−−−...
example 3
DMXAA Activation of IRF-3-Mediated Gene Expression
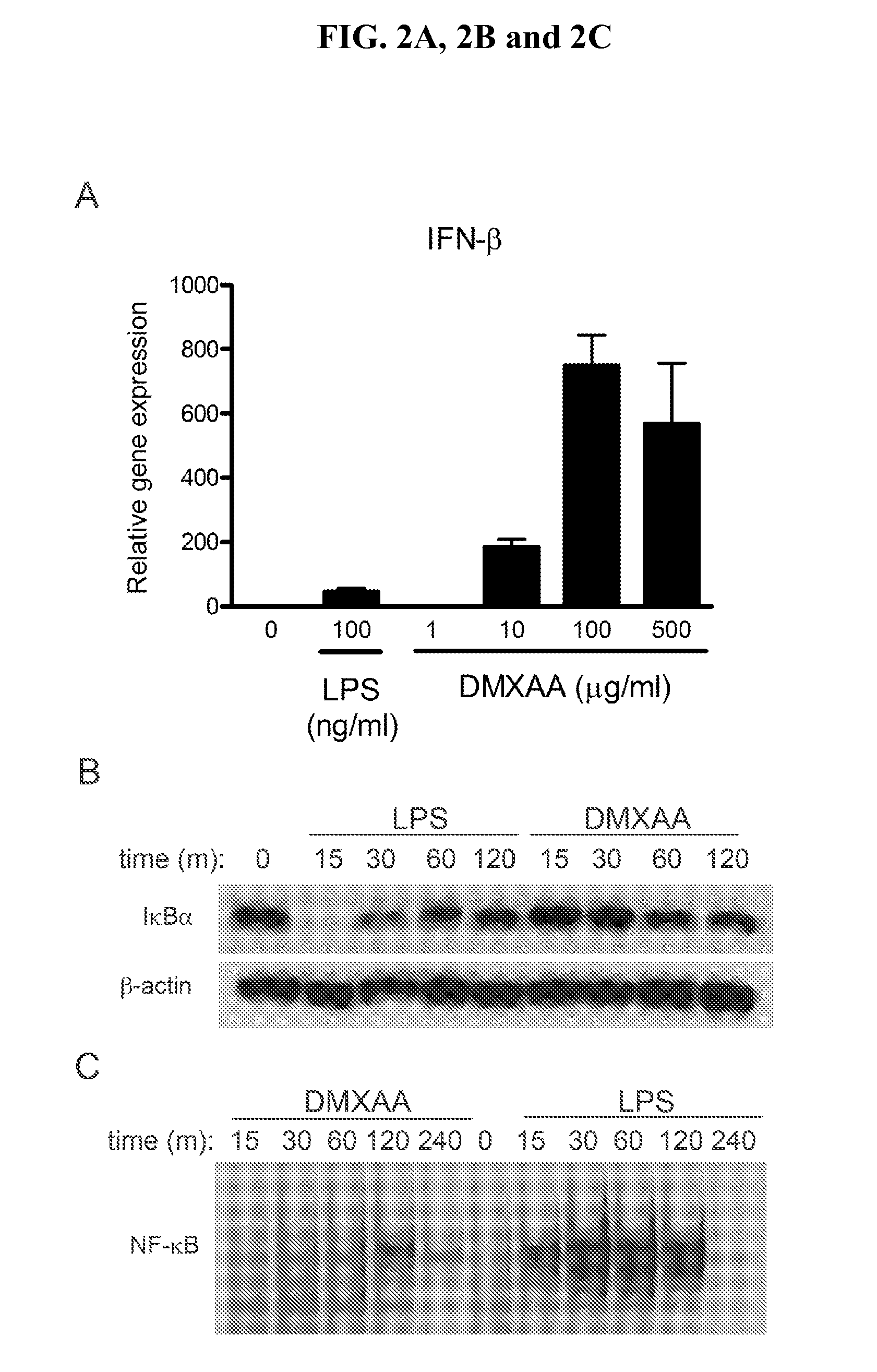
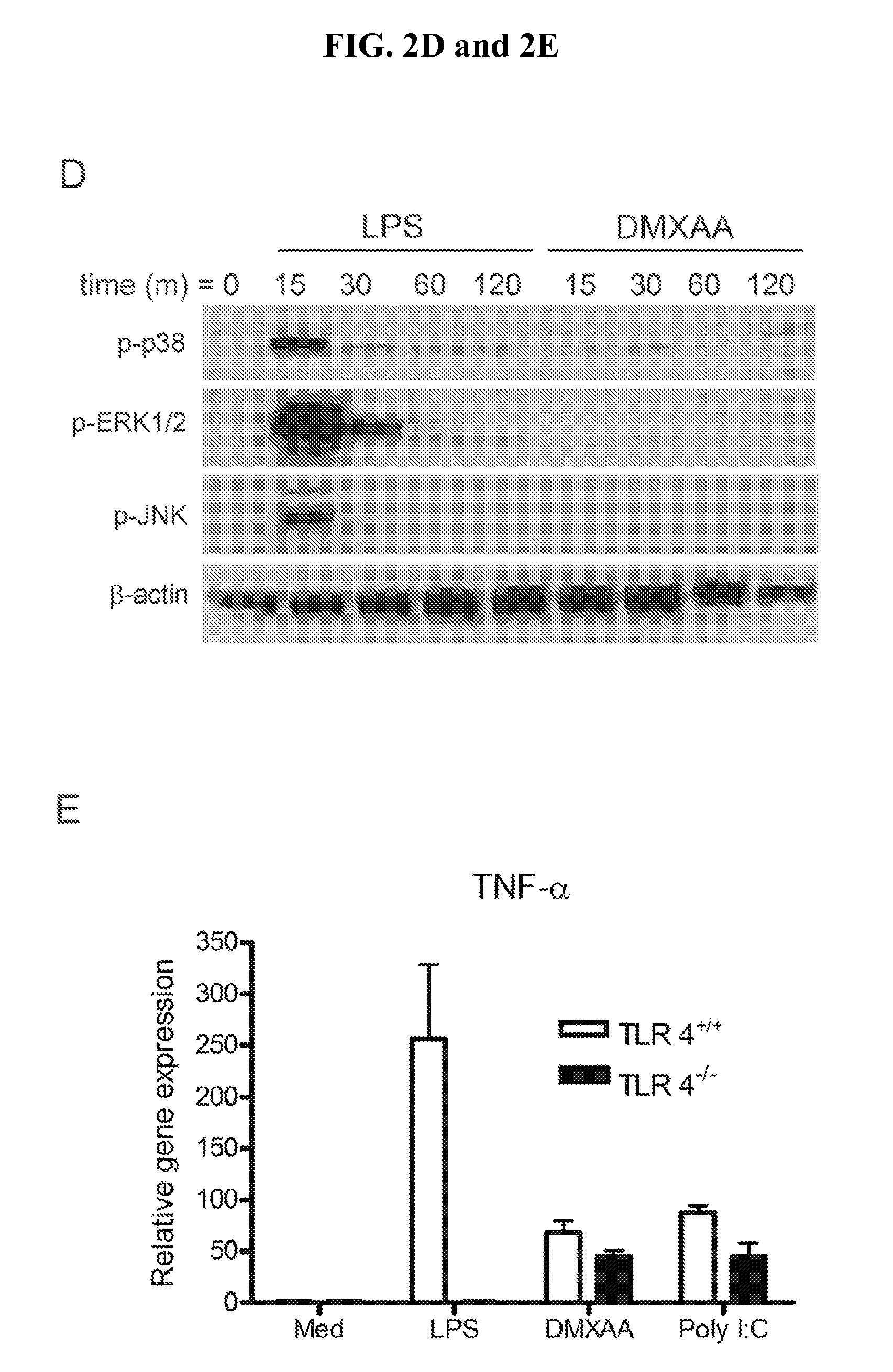
[0091]FIGS. 2A, 2B, 2C, 2D and 2E demonstrate that DMXAA is a much more potent inducer of IFN-β protein and IP-10 mRNA in murine macrophages than LPS, while LPS stimulation results in much higher levels of proinflammatory cytokines, e.g., TNF-α and IL-1β. FIG. 2A shows, using real-time PCR to quantify mRNA expression in peritoneal exudate macrophages, that DMXAA induces 10-fold more IFN-β steady-state mRNA than LPS. While LPS stimulation leads to the rapid disappearance of IκBα (FIG. 2B) and NF-κB translocation (FIG. 2C) in primary macrophages and the RAW 264.7 macrophage-like cell line, respectively, treatment with DMXAA at doses 10-fold above those observed to be saturating in FIG. 2A has little effect on either the level of IκBα protein or NF-κB binding activity. Under conditions where LPS activates p38, ERK, and JNK MAPK in C57BL / 6 macrophages, DMXAA has no discernible effect (FIG. 2D). FIG. 2E shows that LPS fails to induce TNF-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com