Manganese dry battery and manganese dry battery manufacturing method
a technology of dry battery and manganese, which is applied in the direction of cell components, cell components, jackets/cases materials, etc., can solve the problems of lowering battery capacity and discharge characteristics, insufficient suppression occurrence of zinc can corrosion, etc., to suppress the leakage of electrolytic solution, suppress the zinc reaction, and large hydrogen overvoltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
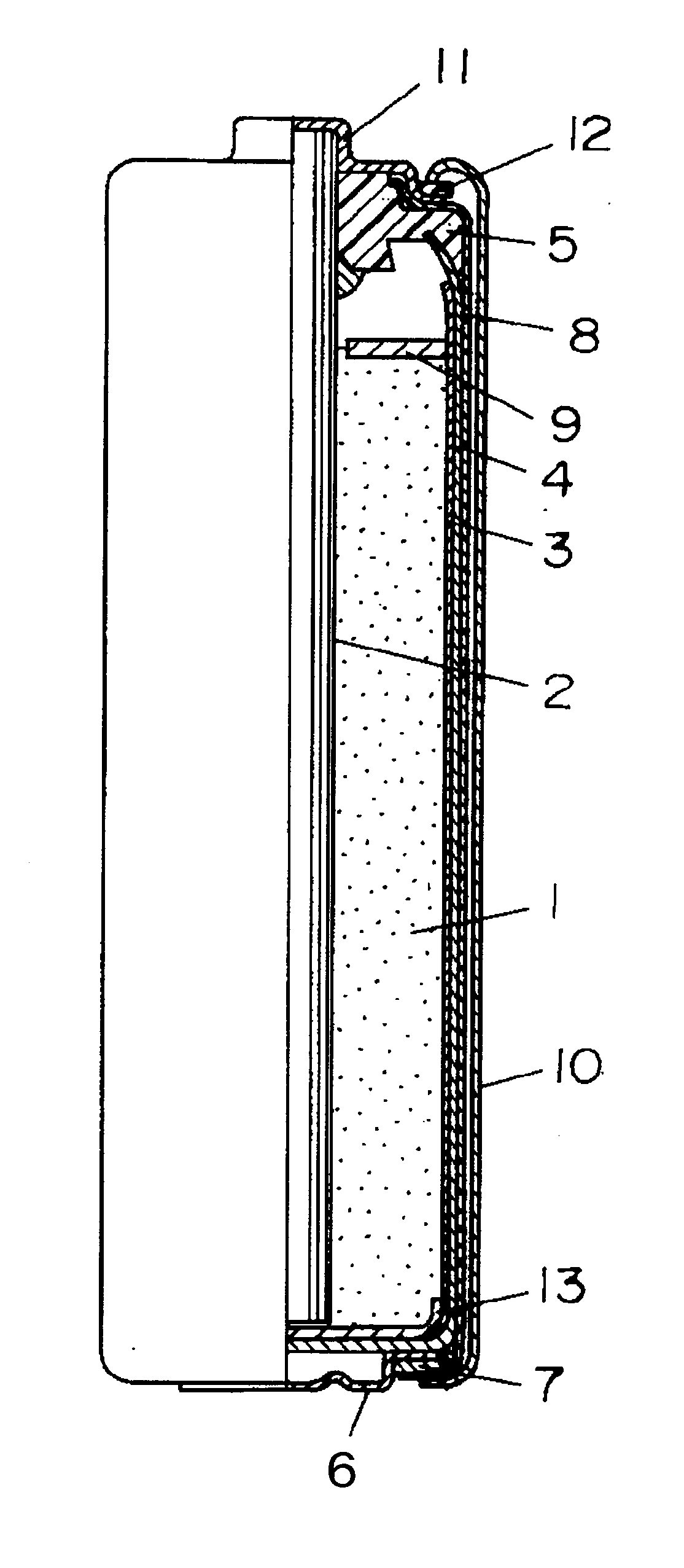
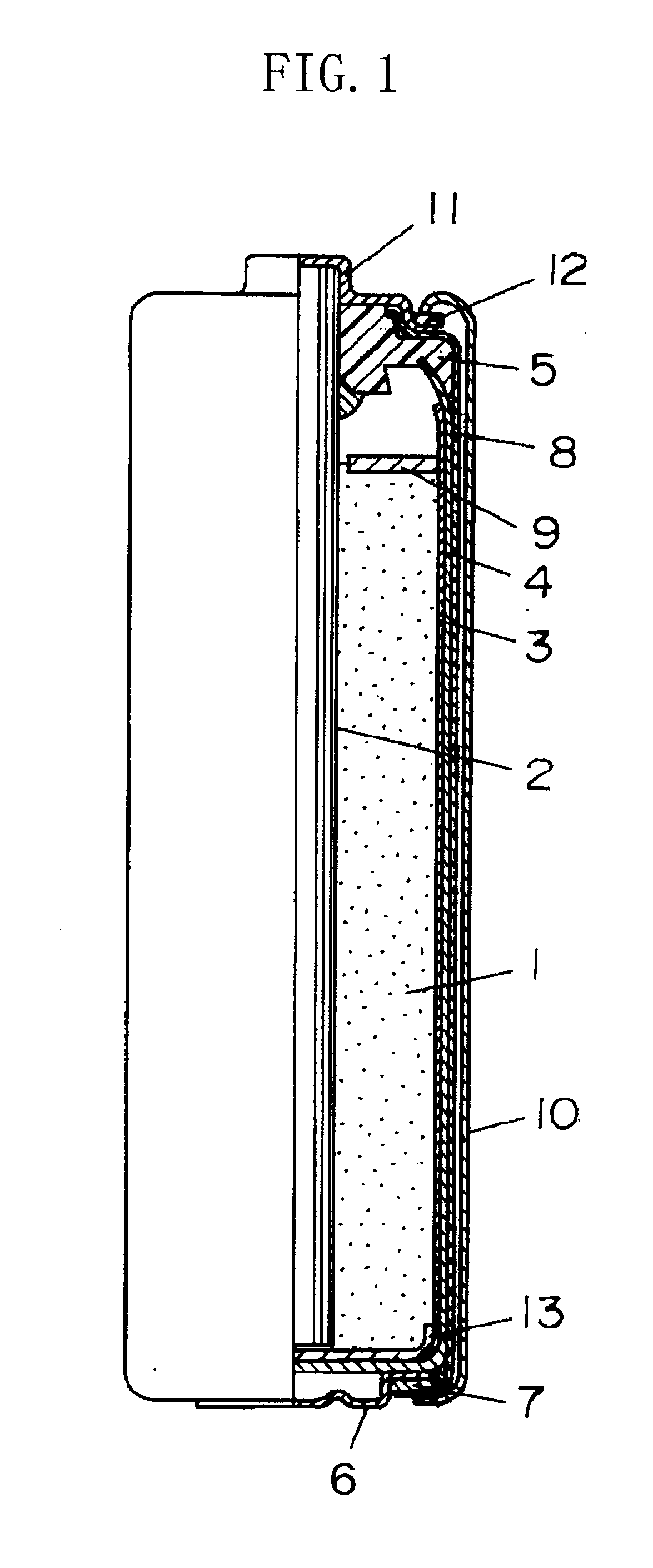
[0028]FIG. 1 is a sectional view showing a structure of a manganese dry battery in accordance with Embodiment 1 of the present invention, wherein a battery can is formed of a negative electrode zinc can 4 in a bottomed cylindrical form.
[0029]As shown in FIG. 1, within the negative electrode zinc can 4, a positive electrode mixture 1 is inserted with a separator 3 containing an electrolytic solution interposed, and a carbon rod 2 as a current collector is inserted in the central part of the positive electrode mixture 1. The upper end opening of the negative electrode zinc can 4 is sealed by a sealant 5. The carbon rod 2 passes through the center hole of the sealant 5 so as to be in contact with a positive electrode 11 while a negative electrode terminal 6 is mounted at the bottom of the negative electrode zinc can 4. The outer peripheral face of the negative electrode zinc can 4 is covered with a plastic tube 8, and a metal jacket 10 is fitted to the outer periphery of the plastic tu...
embodiment 2
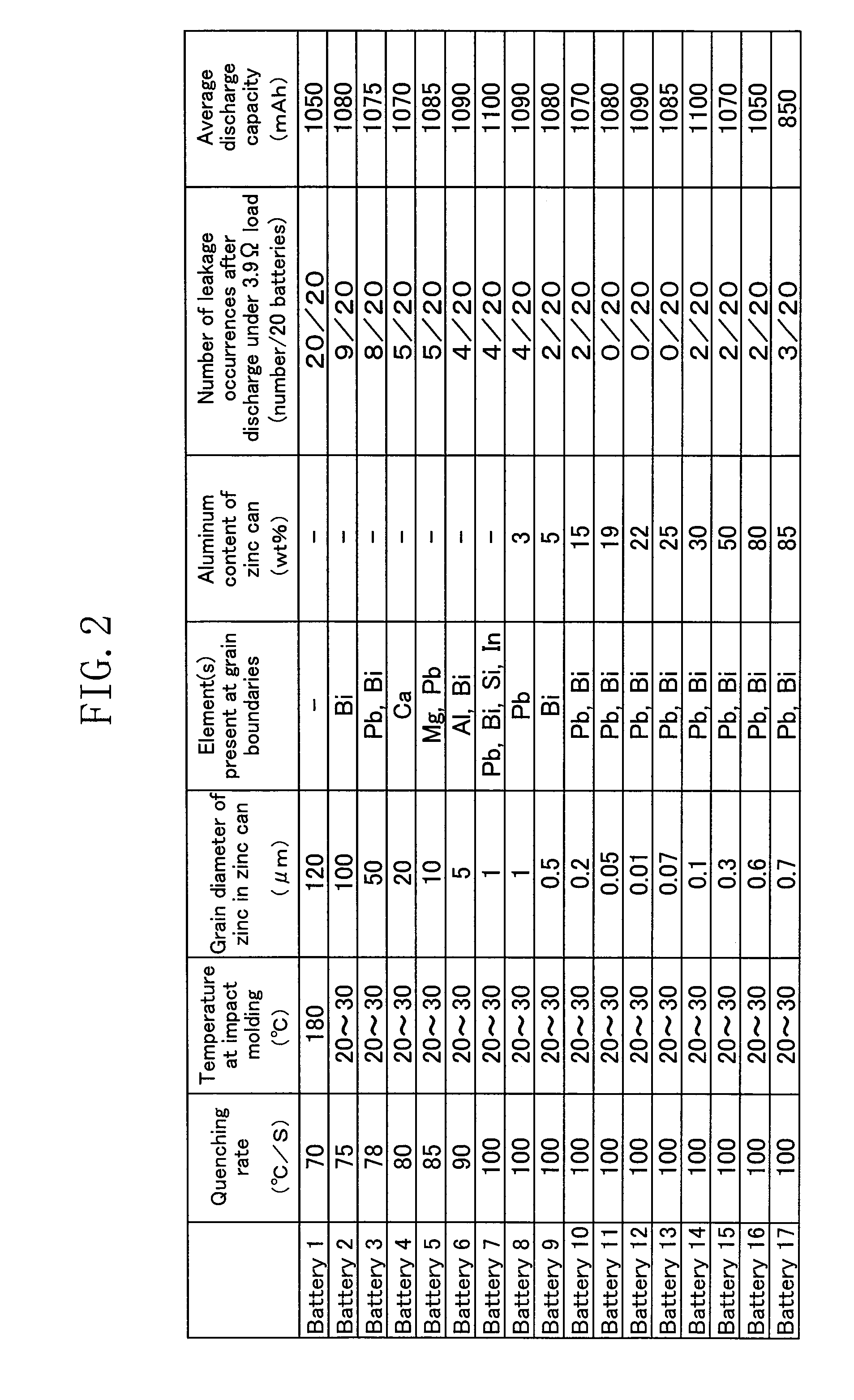
[0038]While yielding of the added element other than zinc at the grain boundaries of zinc uniforms the zinc reaction in Embodiment 1 of the present invention, the zinc reaction can be uniformed by reducing the grain diameter of zinc by one place or more than ever.
[0039]In the present embodiment, a negative electrode zinc can 4 used in a manganese dry battery is formed of a zinc plate obtained by quenching and casting melted zinc containing aluminum of 3 to 80 weight %.
[0040]Quick quenching of the melted zinc containing aluminum of such an amount minimizes the grain diameter of zinc forming the zinc can 4 to approximately 0.01 to 1 μm. This attains a zinc can in which zinc has a grain diameter one place or more smaller than ever. As a result, the zinc reaction is uniformed not only at the surface of the zinc can but also inside the zinc can, thereby effectively suppressing leakage of the electrolytic solution caused due to hole formation in the zinc can.
[0041]When the content of alum...
working examples
[0048]Results obtained by evaluating the corrosion resistance of manganese dry batteries in the present invention will be explained on the basis of the working examples. It is noted that the present invention is not limited to the following working examples.
Battery 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com