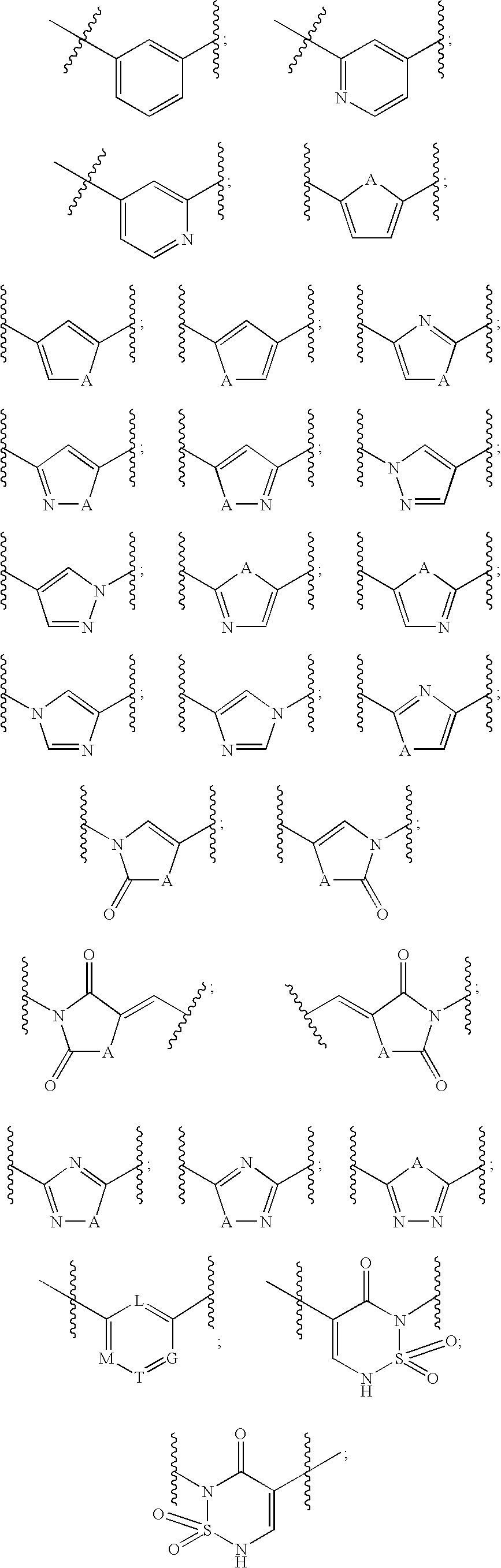
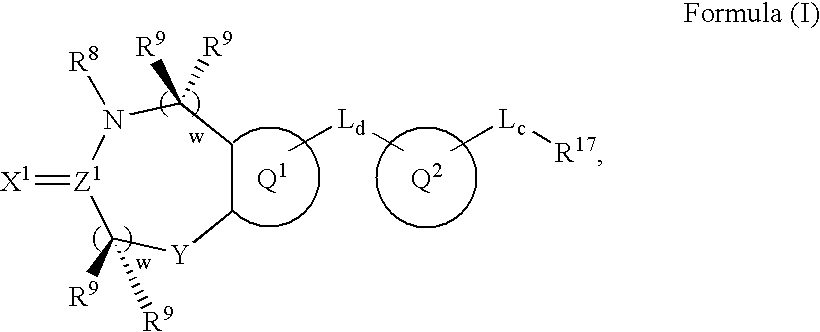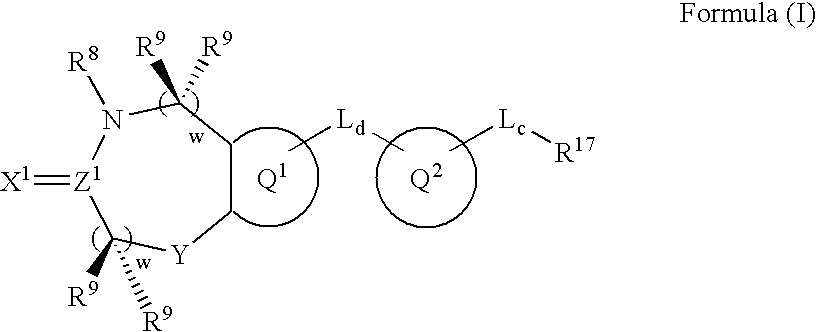Metalloprotease inhibitors containing a heterocyclic moiety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 3a to 3b
Preparative Examples 3a to 3b
[0138]Following a similar procedure as described in the Preparative Example 3, except using the aminoalcohols indicated in Table 1.3 below, the following compounds were prepared.
TABLE I.3Prep. Ex. #aminealcoholproductyield / MS3an.d.[MH]+ = 246 / 483b19%[MH]+ = 246 / 48
example 4
Preparative Example 4
[0139]
Step A
[0140]A suspension of the title compound from the Preparative Example 3, Step B (567 mg) and CuCN (230 mg) in dry N-methyl-pyrrolidin-2-one (15 mL) was degassed under Argon and heated under microwave irradiation to 200° C. for 2 h. The mixture was concentrated, diluted with 1N HCl (100 mL) and extracted with EtOAc (200 mL). The organic layers were washed with H2O (2×200 mL) and brine (200 mL), dried (MgSO4), filtered, absorbed on silica and purified by flash chromatography (cyclohexane / ethyl acetate 7:3 to 6:5) to give the title compound as a colourless solid. [MH]+=211.
Step B
[0141]To an ice cooled solution of the title compound from Step A above in dry MeOH (20 mL) were added di-tert-butyl dicarbonate (500 mg) and NiCl2.6H2O (20 mg), followed by the careful portionwise addition of NaBH4 (320 mg). The resulting black mixture was stirred for 20 min at 0-5° C. (ice bath), then the ice bath was removed and stirring at room temperature was continued over...
examples 4a to 4d
Preparative Examples 4a to 4d
[0143]Following a similar procedure as described in the Preparative Example 4, Step A except using the educt indicated in Table I.4 below, the following compounds were prepared.
TABLE I.4Prep. Ex. #eductproductyield / MS4an.d.[MH]+ = 1934b93%[MH]+ = 1934cn.d.[MH]+ = 1934d55%[MH]+ = 211
Preparative Examples 5a to 5f
[0144]Following a similar procedure as described in the Preparative Example 4, Step B except using the educt indicated in Table I.5 below, the following compounds were prepared.
TABLE 1.5Prep. Ex. #eductproductyield / MS5a32%(3 steps)[MNa]+ = 3195b56%[MNa]+ = 3195c28%(2 steps)[MNa]+ = 3195dn.d.[MNa]+ = 3255e58%[MNa]+ = 3015f64%[MNa]+ = 337
Preparative Examples 6a to 6h
[0145]Following a similar procedure as described in the Preparative Example 4, Step C except using the educt indicated in Table I.6 below, the following compounds were prepared.
TABLE I.6Prep. Ex. #eductproductyield / MS6aquant.[M-Cl]+ = 1976bquant.[M-Cl]+ = 1956cquant.[M-Cl]+ = 1976dquant.[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com