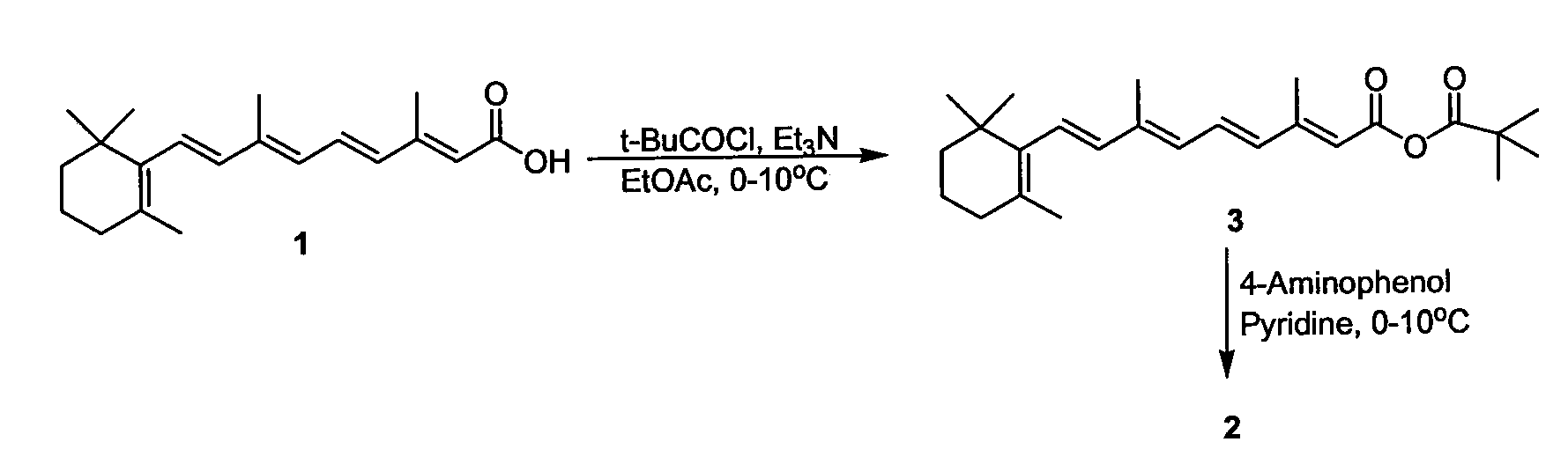
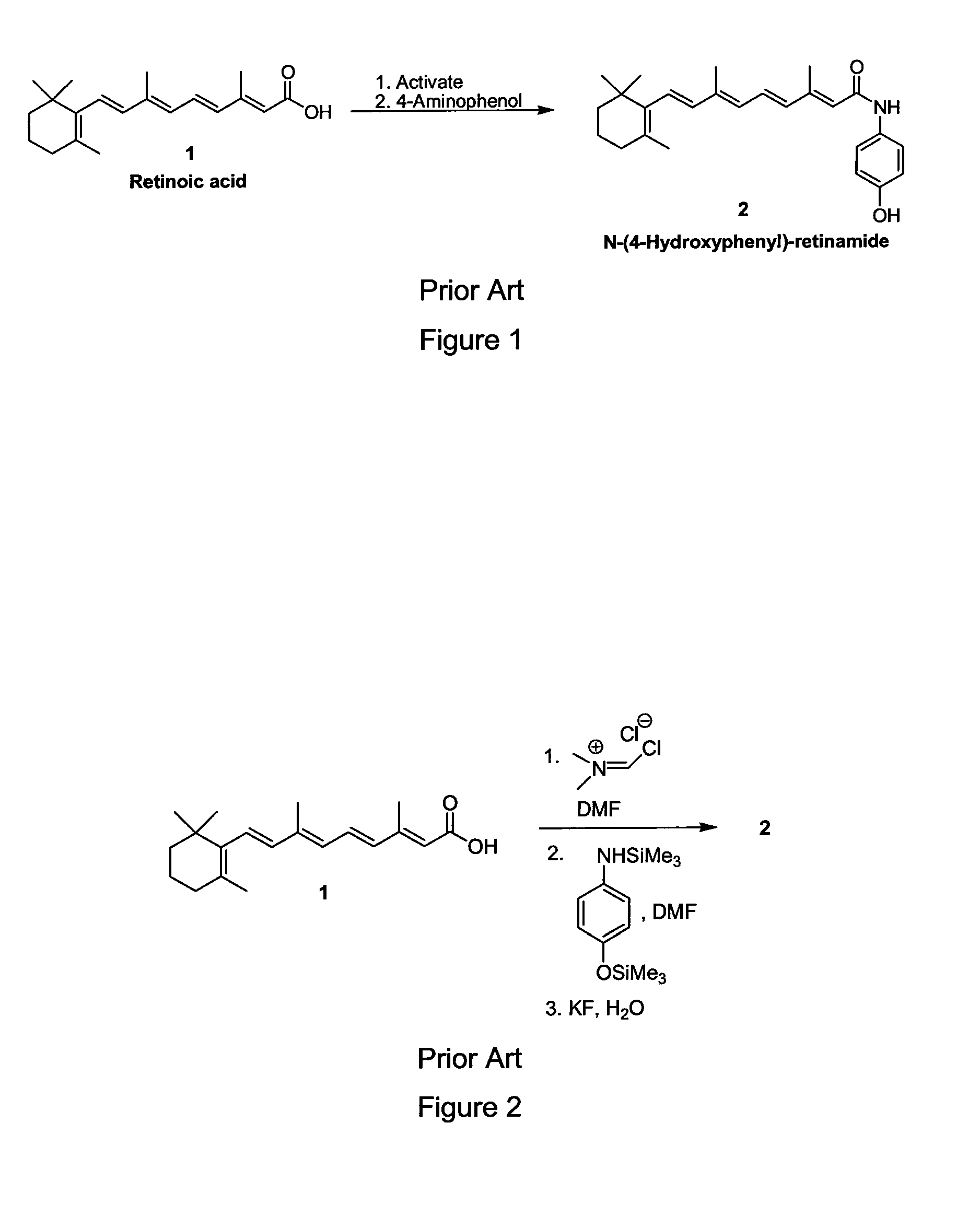
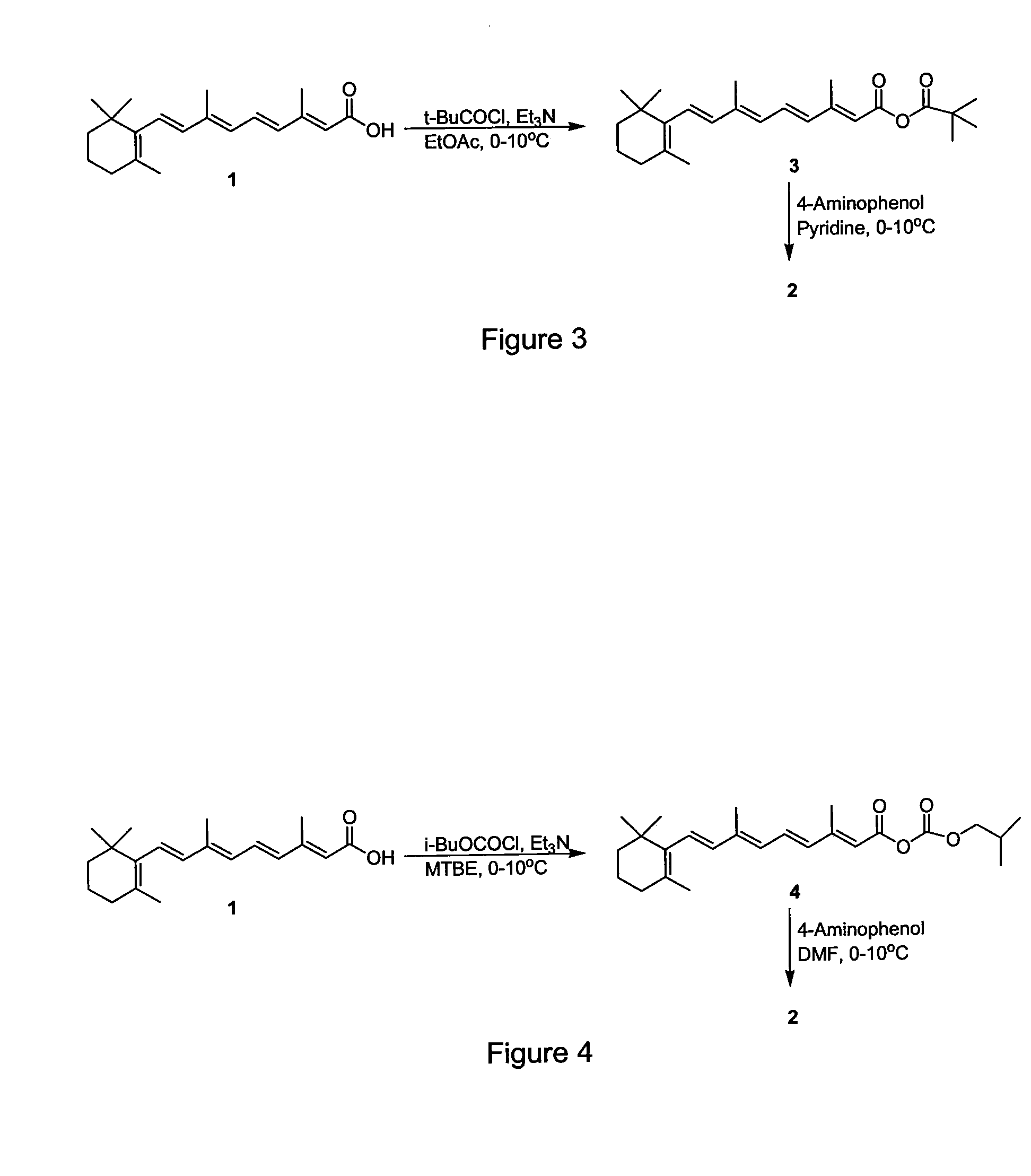
Preparation of Amides of Retinoic Acid Via Mixed Anhydride and Mixed Carbonate Intermediates
a technology of retinoic acid and amides, which is applied in the preparation of carboxylic acid amides, chemistry apparatus and processes, and organic chemistry, etc. it can solve the problems of unsuitable scale-up methods and achieve the effect of simple, scalable and less expensive processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the t-Butyl Mixed Anhydride of Retinoic Acid
[0029]To a 100 milliliter round bottom flask was added 2.00 grams (6.67 mmol) of retinoic acid and 40 milliliters of t-butyl methyl ether. The slurry was cooled to a bath temperature of about 0-10° C. To the slurry was added 1.02 milliliters (7.40 mmol) of triethylamine via syringe over about 1 minute. Trimethylacetyl chloride (0.86 milliliters, 7.0 mmol) was then added via syringe over about 10 minutes. The bright yellow slurry was then stirred for 3 hours at a bath temperature of about 0-10° C. and then held overnight in a refrigerator. The next day the reaction mixture was filtered, the filtered solids were washed thoroughly with t-butyl methyl ether, and the filtrate was concentrated via rotary evaporation (about 35-40° C. external temperature) to give 2.84 grams of the mixed anhydride as a gold oil. The NMR and IR of the oil corresponded to the assigned structure (t-butyl mixed anhydride of retinoic acid). The presence ...
example 2
Preparation of Fenretinide Via the t-Butyl Mixed Anhydride of Retinoic Acid
[0030]To a 5-Liter 4 neck round bottom flask equipped with a mechanical stirrer, temperature probe, addition funnel, and nitrogen inlet adapter was added 100.0 grams (333.3 mmol) of retinoic acid and 1.0 liter of ethyl acetate (EtOAc). The slurry was cooled to an internal temperature of 5° C. Triethylamine (50.7 milliliters, 366 mmol) was added all at once via graduated cylinder. To the thin slurry was then added trimethylacetyl chloride (45.1 milliliters, 366 mmol) via addition funnel over 23 minutes at an internal temperature of less than 5° C. After 3.5 hours, the reaction was judged to be complete by HPLC analysis. To the bright yellow slurry of the mixed anhydride was added a thin suspension of 4-aminophenol (76.3 grams, 700 mmol) in 500 milliliters of pyridine over 30 minutes at an internal temperature of 3-5° C. After 2 hours, the reaction was judged to be complete by HPLC analysis. To the slurry was a...
example 3
Preparation of Fenretinide Using 2,2-Dimethylbutyrlchloride
[0032]To a 250 milliliter round bottom flask was charged 2.00 grams (6.67 mmol) of retinoic acid and 20 milliliters of CH3CN. The slurry was cooled to an external temperature of 0-10° C. while 1.0 milliliters of Et3N (7.3 mmol) was charged via syringe. To the slurry was added 0.96 milliliters (7.0 mmol) of 2,2-dimethylbutyrlchloride via syringe over about 10 minutes. A thick, unstirrable slurry formed. Ten milliliters of additional CH3CN was added to improve the stirring. After about 3.5 hours, the activation was judged to be complete by HPLC analysis. To the slurry was added 2.0 milliliters of pyridine via syringe. 4-aminophenol (1.53 grams, 14.0 mmol) was then added as a solid in 2 portions over about 15 minutes. After stirring for 2 hours, the reaction was held overnight in the refrigerator (about 0-10° C.). The following morning the reaction was judged to be complete by HPLC analysis. At an external temperature of 0-10° ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com