Cytotoxic depsipeptides
a cytotoxic and peptide technology, applied in the field of new depsipeptide compounds, can solve the problems of cancer being the leading cause of death in animals and humans
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of IB-01211
[0080] Inoculum development: a frozen culture of ES7-008 or a well grown slant culture (5% vol.) is used to seed 100 ml of a seed medium, as described in Table 1, that it is contained in a 250 ml shake flask. The flask is incubated during 48 h. A 2 l Erlenmeyer flask with 500 ml of the same medium is seeded with 10% vol. of the first stage inoculum. The flask is incubated during 48 h.
[0081] Fermentation step: 50 l of production medium, as described in Table 1, contained in a 75 l fermentation tank are seeded with 2.5 l of second stage inoculum. The fermentation is carried out during 96 h with 400 rpm agitation and an air flow of 0.5V / V.M.
example 2
Isolation of IB-01211
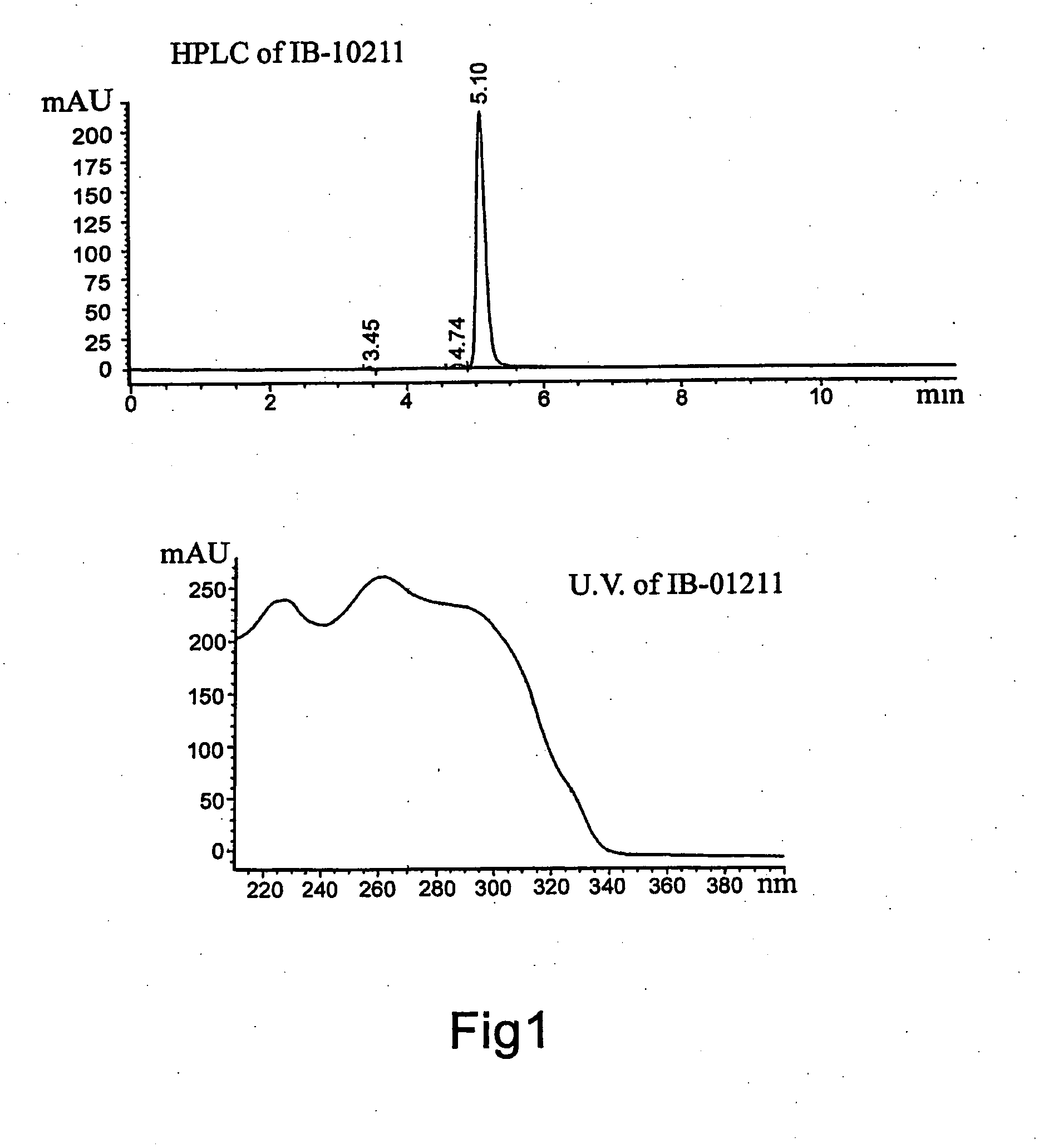
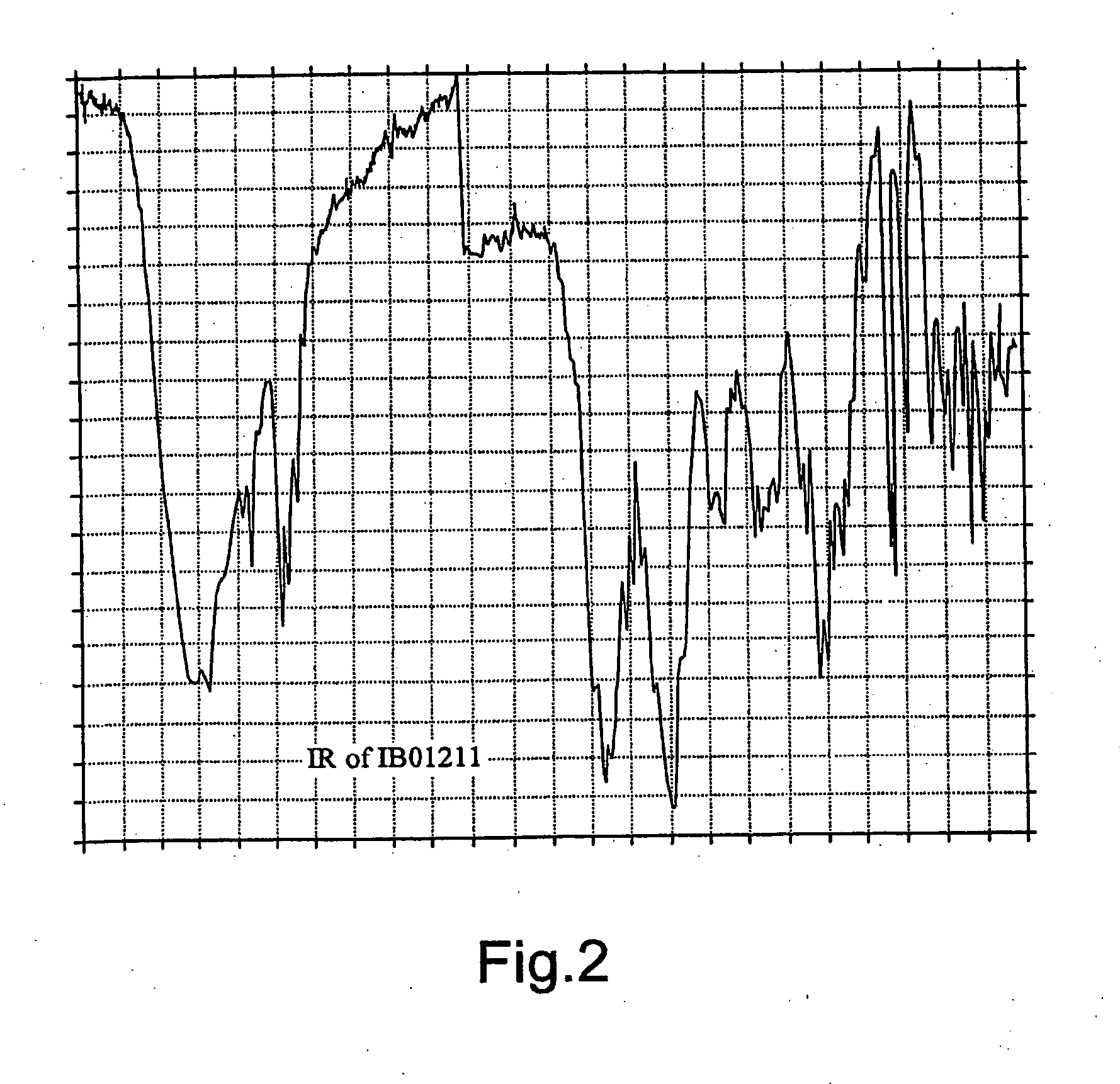
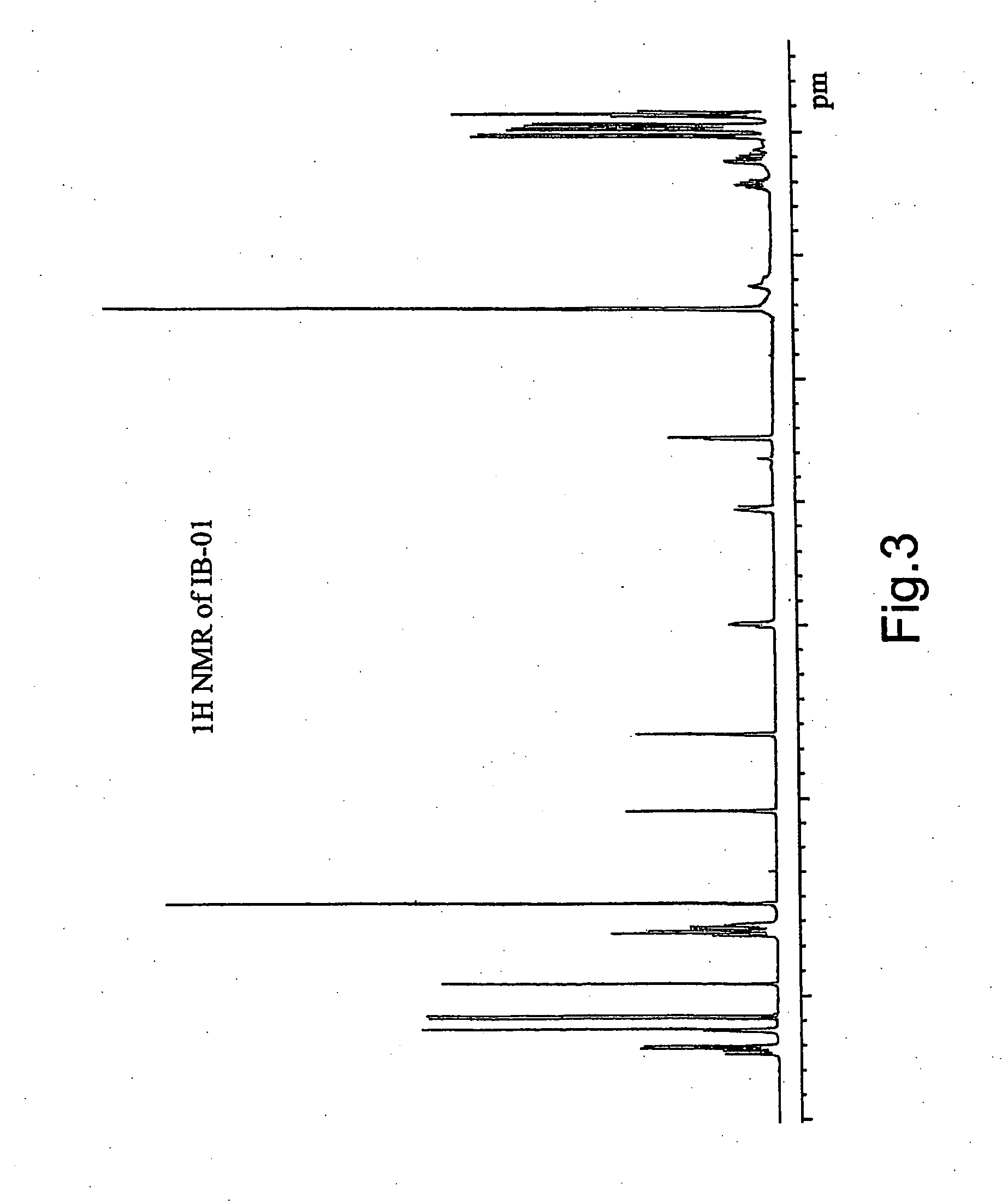
[0082] 8.5 liters of whole harvested broth were filtrated to separate the biomass and other solids. The mycelia cake was extracted twice with a mixture solvent (2.4 l) of CHCl3:CH3OH:H2O (2:1:1). The activity was concentrated in the lower layer. The organic solvent was concentrated and evaporated to dryness in vacuo to yield 4.8 g of crude extract.
[0083] The extract was applied to a silica gel VFC (vacuum flash chromatography) system, using a mixture of n-hexane-EtOAc and EtOAc-MeOH as eluting solvents. The fractions with antitumour activity, containing IB-01211 (900 mg) were eluted with EtOAc-MeOH 1:1, EtOAc-MeOH 1:3 and methanol. The active fractions were chromatographied twice with a silica gel column using CHCl3-MeOH and EtOAc-MeOH mixtures as eluting solvents. The cytotoxic activity was detected in fractions eluted with CHCl3-MeOH 96:4 in the first chromatography (200 mg of pure compound IB-01211) and in fractions eluted with EtOAc-MeOH 85:15-8:2 in the s...
example 3
Biological in vitro Activity Bioassays for Antitumour Screening
[0085] The finality of these assays is to interrupt the growth of an “in vitro” tumour cell culture by means a continued exhibition of the cells to the sample to be testing. The following human cell lines were used:
CELL LINESNameNo ATCCTissueCharacteristicsK-562CCL-243leukemiaerythroleukemia (pleural effusion)A-549CCL-185lunglung carcinoma “NSCL”SK-MEL-28HTB-72melanomamalignant melanomaHT-29HTB-38coloncolon adenocarcinomaDU-145HTB-81prostateprostate carcinoma, not androgenreceptorsLNCaPCRL-1740prostateprostate adenocarcinoma, with androgenreceptorsPC-3CRL-1435prostateprostate adenocarcinomaBT-474HTB-20breastbreast adenocarcinomaMX-1breastbreast adenocarcinoma,Hs746tHTB-135gastricstomach carcinomaSK-HEP-1HTB-52liverliver adenocarcinomaSK-OV-3HTB-77ovaryovary adenocarcinoma (malignant ascites)PANC-1CRL-1469pancreaspancreatic epitheloid carcinoma5637HTB-9bladderbladder carcinomaFADUHTB-43pharynxsquamous cell carcinoma786...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| UV spectrum | aaaaa | aaaaa |
| spectrum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com